FILM DRESSING, semi-permeable, adhesive, 15cmx10m, roll
STD
SDREFIDSR1510
Valid Article
Account code:
60210
HS Code:
300590
Last Updated on:
13/08/2025, 22:10:11
Former
Code(s):
-X SDREFIDS+++
Single use
A single-use device, also referred to as a disposable device, is intended for use on one patient during a single procedure. It is not intended to be reprocessed (i.e., cleaned and disinfected or sterilized).
CE marking: declaration that the product meets EU standards for health, safety, and environmental protection. The CE marking indicates that the product may be sold freely in any part of the European Economic Area, regardless of its country of origin.
M04010202 - Polyurethane fixing dressings
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes
Thermosensitive codes are defined for storage and transportation temperature requirements of the products.
The product is part of at least one Kit.
A kit is a collection of products (medical and/or logistic) that are needed for a certain intervention in emergency. The choice and quantity of the articles reflects the MSF protocols for this specific situation. The use of Kits allows to start an intervention without a detailed evaluation.
FILM DRESSING, semi-permeable, adhesive, roll
Definition
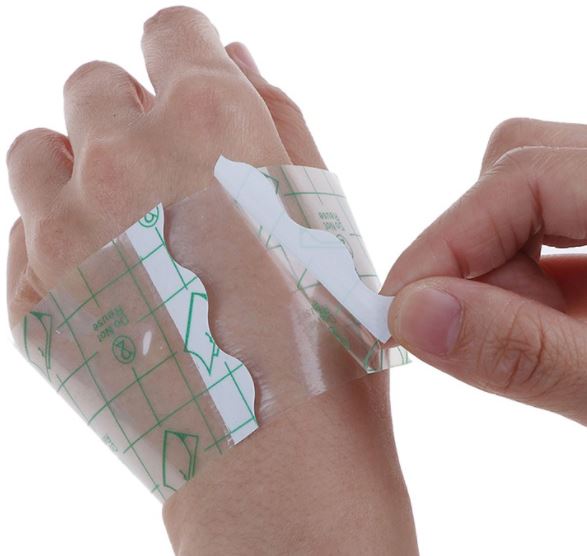
A non-sterile, transparent, semi-permeable (i.e., impermeable to fluids, permeable to vapours and gases) covering applied to skin typically intended to fix a primary dressing or a medical device, or to provide protection from fluids. It is a thin, clear film made of synthetic polymer material that is covered on one side with a pressure-sensitive adhesive. This is a single-use device.
Specifications
Quality standards
EN 13726-2, 2002, Test methods for primary wound dressings. Moisture vapour transmission rate of permeable film dressings
Technical specifications
- transparent film made of synthetic polymer (e.g., polyurethane based), latex- and PVC-free
- breathable: allows moisture vapour and gas exchanges
- moisture vapour transmission rate ≥ 500g/m²/24h
- waterproof: impervious to liquids and body fluids
- protection against all external contamination
- barrier against bacteria and viruses ≥20nm
- adhesive:
- must adhere to the skin during 7 days minimum
- hypoallergenic latex-free
- without anti-microbial agents
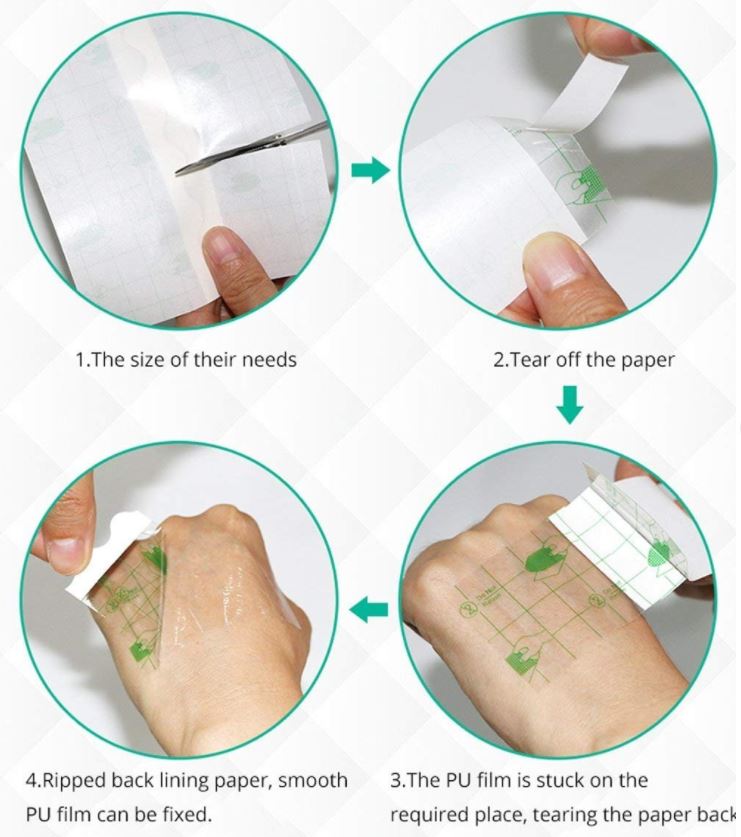
- easy to be cut into shape
- non sterile, roll presentation
- sizes
- length: +/- 10m
- width:
- SDREFIDSR1510: 15 cm
- SDREFIDSR1010: 10 cm
Packaging & Labelling
Unit packaging
Instructions for use
Precautions for Use
- To ensure adequate dressing adhesion, the skin around the wound / puncture site should be clean, dry and free of moisturisers and grease.
- Caution is required when removing the dressing: stretch the film horizontally away from the wound to avoid damage to the skin.
Storage
- Store below 25°C
- Protect from sunlight ‐ Protect from humidity
MSF requirements
Transparent polyurethane film dressing, easy to cut, allowing the protection of a wound or puncture site against liquids and colonization by germs from the outside
Some restricted information has been hidden. Sign in
to see this information







![[KMEDMHWS31-] (mod ward) MEDICAL RENEWABLE SUPPLIES 2021](/web/image/product.template/574357/image_256/%5BKMEDMHWS31-%5D%20%28mod%20ward%29%20MEDICAL%20RENEWABLE%20SUPPLIES%202021?unique=4d33748)
![[KMEDMHES35-] (mod emergency) CATHETERS, TUBES & DRAINS 2021](/web/image/product.template/574355/image_256/%5BKMEDMHES35-%5D%20%28mod%20emergency%29%20CATHETERS%2C%20TUBES%20%26%20DRAINS%202021?unique=0590e60)