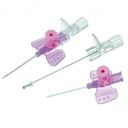
SAFETY IV CATHETER, tip, 22G x 25 mm, wings, IP, blue
Valid Article
SAFETY IV CATHETER, tip shield with IP
Definition
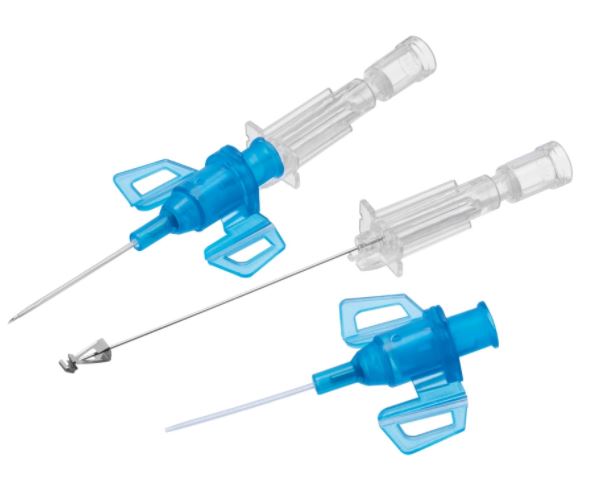
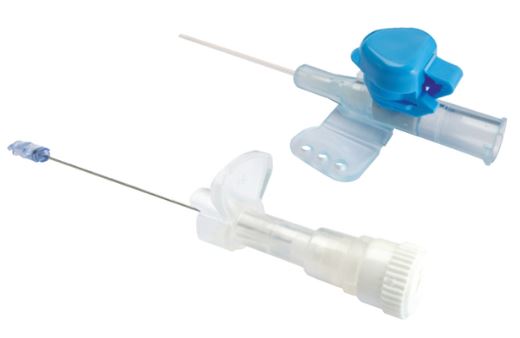
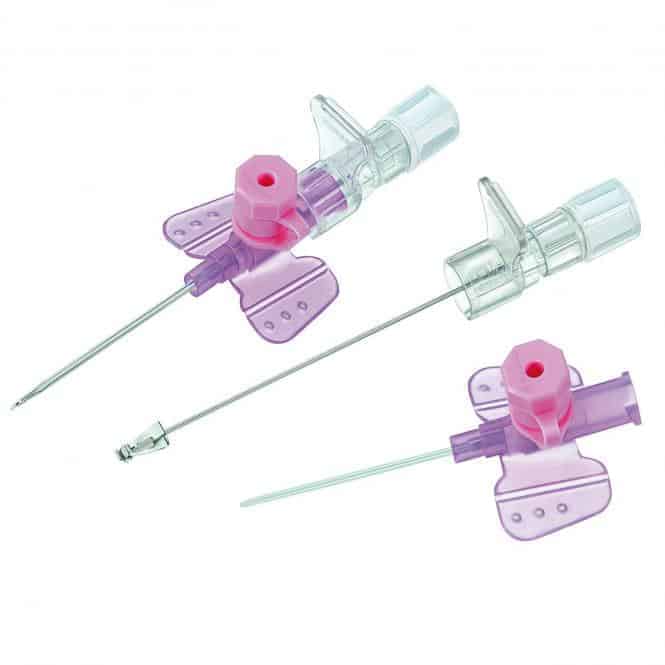
A sterile, thin, flexible tube intended to be inserted into the peripheral vasculature of a patient to enable short-term (< 30 days) intravascular access. It is not intended to be advanced to the central vasculature. It includes a stylet for catheter introduction, an injection port and wings for fixation.
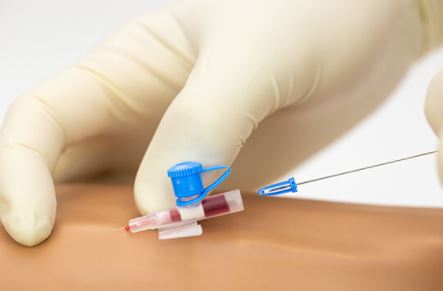
It is a safety injection device reducing the risk of needle stick injuries. It may be used to administer fluids/blood or medication.
Specifications
Quality standards
- EN ISO 10555-1, 2013, edition 3, +A1 2017 Sterile, single-use intravascular catheters - Part 1: General requirements
- ISO 10555-5, 2013, edition 2, (confirmed 2018) Sterile, single-use intravascular catheters - Part 5: Over-needle peripheral catheter
- ISO 23908, 2013, edition 1, Sharps injury protection - Requirements and test methods - Sharps protection features for single-use hypodermic needles, introducers for catheters and needles used for blood sampling
Technical specifications
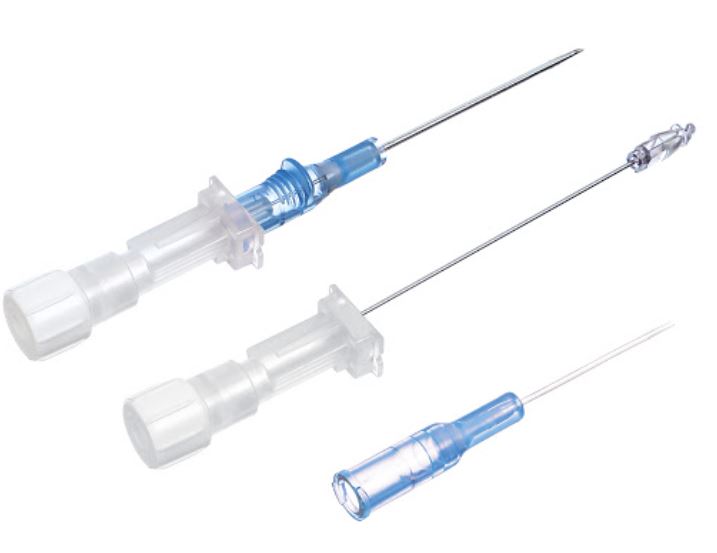
- CATHETER
- polyurethane (PUR) is the first choice material, FEP is the second choice option.
- straight and tapered distal end
- translucent hub with standardized colour code (ISO 10555-5) and female Luer connector
- fitted with wings (Butterfly)
- with injection port
- STYLET
- stainless steel
- slightly longer than the cannula
- bevelled end
- translucent hub (flashback chamber) to observe blood reflux
- fitted with a stopper (flashplug)
- PROTECTIVE SHEATH
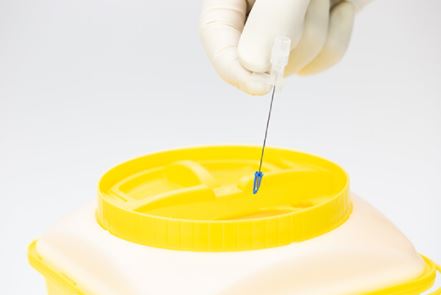
- SAFETY MECHANISM
- While retracting the stylet from the catheter, a device is placed upon the tip of the stylet (shape of tip is brand specific, see picture)
- Passif safety mechanism: the user does not have to activate the safety mechanism.
- The safety tip protection remains in place once activated.
- Sterile, for single use
Comes in 6 sizes: 14G to 24G
Caution, the colour code used to denote Gauge is different from that used for other needles.
SEE TABLE BELOW
Packaging & Labelling
Unit sterile packaging in peel-open pack
Instructions for use
Please consult the Manual of nursing Care Procedures, MSF, 2020 available online via the Nursing care working Group sharepoint page.
MSF requirements
IV catheter with passive safety mechanism for general use.
for the 24G catheter, the model without injection port is preferred (SINSIVCST24W2)
Extra Tables
Colour / Couleur | Gauge | External Ø | Internal Ø | length / longueur | flow rate ml/min | Use / utilisation |
Yellow Jaune | 24 | 0.74 mm | 0.55 mm | 19 mm | 23 | neonatology, paediatrics néonatalogie, pédiatrie |
Blue Bleu | 22 | 0.90 mm | 0.65 mm | 25 mm | 36 | infusion in veins of reduced calibre, extremities of age perfusion dans des veines de calibre réduit, âges extrêmes |
Pink Rose | 20 | 1.00 mm | 0.75 mm | 32 mm | 60 | typical infusion / perfusion classique |
Green Vert | 18 | 1.30 mm | 0.95 mm | 45 mm | 95 | transfusion |
Grey Gris | 16 | 1.75 mm | 1.35 mm | 45 mm | 200 | emergency, fluid resuscitation requires at least 16 G cannula urgence, la réanimation liquidienne nécessite au moins une canule de 16 G |
Orange | 14 | 2.1 mm | 1.60 | 45 mm | 305 | emergency, rapid transfusion/infusion, large volumes urgence, transfusion/perfusion rapide, gros volumes |




![[KMEDKFAI5RS] MOBILE MEDICAL BAG KIT, rucksack](/web/image/product.template/572106/image_256/%5BKMEDKFAI5RS%5D%20MOBILE%20MEDICAL%20BAG%20KIT%2C%20rucksack?unique=5ba81ea)
![[KMEDMHCS141] (mod OPD) COMPLEMENTARY INJECTION SUPPLIES](/web/image/product.template/572723/image_256/%5BKMEDMHCS141%5D%20%28mod%20OPD%29%20COMPLEMENTARY%20INJECTION%20SUPPLIES?unique=9283e96)
![[KMEDMNUTI33] (module nut. inpatient) MEDICAL SUPPLIES 2021](/web/image/product.template/575289/image_256/%5BKMEDMNUTI33%5D%20%28module%20nut.%20inpatient%29%20MEDICAL%20SUPPLIES%202021?unique=ce93f30)
![[KMEDMNUTO33] (module nut. outpatient) MEDICAL SUPPLIES 2021](/web/image/product.template/575294/image_256/%5BKMEDMNUTO33%5D%20%28module%20nut.%20outpatient%29%20MEDICAL%20SUPPLIES%202021?unique=5c1c774)
![[KMEDMHDS21-] (mod delivery & neonate) RENEWABLE SUPPLIES compl. 2019](/web/image/product.template/569223/image_256/%5BKMEDMHDS21-%5D%20%28mod%20delivery%20%26%20neonate%29%20RENEWABLE%20SUPPLIES%20compl.%202019?unique=5c1c774)
![[KMEDMHIS24-] (mod ICU) INJECTION SUPPLIES 2021](/web/image/product.template/574424/image_256/%5BKMEDMHIS24-%5D%20%28mod%20ICU%29%20INJECTION%20SUPPLIES%202021?unique=aecada5)
![[KMEDMHOS33-] (mod OT Room) INJECTION SUPPLIES 2021](/web/image/product.template/574470/image_256/%5BKMEDMHOS33-%5D%20%28mod%20OT%20Room%29%20INJECTION%20SUPPLIES%202021?unique=e4f6df6)
![[KMEDMSUP05M] (IEHK 2024 suppl. module) SUPPLEMENTARY MALARIA UNIT](/web/image/product.template/583180/image_256/%5BKMEDMSUP05M%5D%20%28IEHK%202024%20suppl.%20module%29%20SUPPLEMENTARY%20MALARIA%20UNIT?unique=fa0ffa3)
![[KMEDMSUP05S] (IEHK 2024 suppl. module) SUPPLEMENTARY RENEWABLE UNIT](/web/image/product.template/583185/image_256/%5BKMEDMSUP05S%5D%20%28IEHK%202024%20suppl.%20module%29%20SUPPLEMENTARY%20RENEWABLE%20UNIT?unique=fa0ffa3)
![[KMEDMHCS14-] (mod OPD) INJECTION SUPPLIES](/web/image/product.template/572711/image_256/%5BKMEDMHCS14-%5D%20%28mod%20OPD%29%20INJECTION%20SUPPLIES?unique=9ad44fb)
![[KMEDMHHS24-] (mod hospital) INJECTION SUPPLIES](/web/image/product.template/572826/image_256/%5BKMEDMHHS24-%5D%20%28mod%20hospital%29%20INJECTION%20SUPPLIES?unique=9ad44fb)
![[KMEDMCHO021] (module 001) RENEWABLE SUPPLIES 2019](/web/image/product.template/569222/image_256/%5BKMEDMCHO021%5D%20%28module%20001%29%20RENEWABLE%20SUPPLIES%202019?unique=af26664)