NEEDLELESS CONNECTOR, split-septum, neutral, sterile, s.u.
STD
SINSNLVCSN1
Valid Article
Account code:
60210
HS Code:
901839
Last Updated on:
30/06/2025, 01:02:36
Single use
A single-use device, also referred to as a disposable device, is intended for use on one patient during a single procedure. It is not intended to be reprocessed (i.e., cleaned and disinfected or sterilized).
CE marking: declaration that the product meets EU standards for health, safety, and environmental protection. The CE marking indicates that the product may be sold freely in any part of the European Economic Area, regardless of its country of origin.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes
Thermosensitive codes are defined for storage and transportation temperature requirements of the products.
The product is part of at least one Kit.
A kit is a collection of products (medical and/or logistic) that are needed for a certain intervention in emergency. The choice and quantity of the articles reflects the MSF protocols for this specific situation. The use of Kits allows to start an intervention without a detailed evaluation.
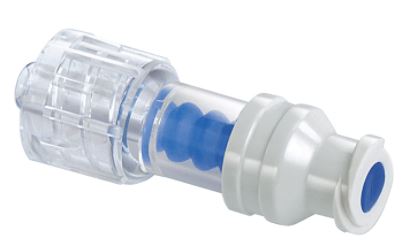
NEEDLELESS CONNECTOR, split-septum, neutral
Definition
A small, sterile, stand-alone, needleless plastic valve intended to mate two related intravenous line devices (e.g., hypodermic syringe and catheter port or tubing from an IV administration set) and hold them in a secured, sealed, locked position, and through which IV access is gained. It is intended to eliminate the use of needles for IV administration of medications.
Specifications
Quality standards
ISO 8536-12, 2021, edition 2, Infusion equipment for medical use - Part 12: Check valves for single use
Technical specifications
Neutral: designed to prevent reflux into catheter or tubing with connection or disconnection (possible small amount, 0.02ml, of reflux may occur)
- Luer activated
- Smooth septum surface (no valleys, no crevices)
- Tight seal between septum and housing
- Straight fluid path
- Split septum valve: a prepierced septum that can be of blunt cannula or Luer-lock design
- Minimal residual volume (< 0.2 ml)
- Clear housing to visualise flushing
- Compatible with all peripheral and central catheters, with infusion pump sets and with blood products
- Plastic polycarbonate, latex free
- No antimicrobial coating
- Sterile, for single use
Packaging & Labelling
Unit sterile packaging in peel-open pack
Instructions for use
- Do not over-tighten a Luer beyond the friction fit as this may damage both the Luer and the connector.
- Do not hold catheter hub during disconnect as this may cause accidental removal of connector from hub.
- After blood use, the connector can be flushed clean and does not require change-out.
- Use routine flushing if connector is not used: 10 ml normal saline / 24 hours in order to maintain catheter patency.
- Change connector in accordance with facility protocol and CDC guidelines: No more frequently than at 96-hour intervals, but at least every 7 days.
MSF requirements
For use with central venous catheters and PICC lines.
For use with peripheral IV catheters in specific contexts (such as VHF)
Some restricted information has been hidden. Sign in
to see this information









![[DEXTCHLHA2W] CHLORHEXIDINE 2%, 70% isopropyl alcohol, SWAB/WIPE](/web/image/product.template/570781/image_256/%5BDEXTCHLHA2W%5D%20CHLORHEXIDINE%202%25%2C%2070%25%20isopropyl%20alcohol%2C%20SWAB-WIPE?unique=3cd4694)
![[SINSCVCAD408] CENTRAL VENOUS CATHETER SET, 2 lumens, CH4-4.5 x 8-10cm](/web/image/product.template/573036/image_256/%5BSINSCVCAD408%5D%20CENTRAL%20VENOUS%20CATHETER%20SET%2C%202%20lumens%2C%20CH4-4.5%20x%208-10cm?unique=3b8ee57)
![[SINSCVCAD511] CENTRAL VENOUS CATHETER SET, 2 lumens, CH5-5.5 x 10-13cm](/web/image/product.template/573002/image_256/%5BSINSCVCAD511%5D%20CENTRAL%20VENOUS%20CATHETER%20SET%2C%202%20lumens%2C%20CH5-5.5%20x%2010-13cm?unique=3b8ee57)
![[SINSCVCAT720] CENTRAL VENOUS CATHETER SET, 3 lumens, CH7-7.5 x 15-20cm](/web/image/product.template/574712/image_256/%5BSINSCVCAT720%5D%20CENTRAL%20VENOUS%20CATHETER%20SET%2C%203%20lumens%2C%20CH7-7.5%20x%2015-20cm?unique=bb706be)
![[SINSPICC3S1] PICC, CH3, single lumen, catheter+ accessories, sterile,s.u.](/web/image/product.template/572559/image_256/%5BSINSPICC3S1%5D%20PICC%2C%20CH3%2C%20single%20lumen%2C%20catheter%2B%20accessories%2C%20sterile%2Cs.u.?unique=7c387b8)
![[SINSPICC5D1] PICC, CH5, double lumen, catheter+ accessories, sterile,s.u.](/web/image/product.template/572919/image_256/%5BSINSPICC5D1%5D%20PICC%2C%20CH5%2C%20double%20lumen%2C%20catheter%2B%20accessories%2C%20sterile%2Cs.u.?unique=cc2268e)
![[SINSPICC5S1] PICC, CH5, single lumen, catheter+ accessories, sterile,s.u.](/web/image/product.template/572554/image_256/%5BSINSPICC5S1%5D%20PICC%2C%20CH5%2C%20single%20lumen%2C%20catheter%2B%20accessories%2C%20sterile%2Cs.u.?unique=7c387b8)
![[KMEDMHIS24-] (mod ICU) INJECTION SUPPLIES 2021](/web/image/product.template/574424/image_256/%5BKMEDMHIS24-%5D%20%28mod%20ICU%29%20INJECTION%20SUPPLIES%202021?unique=4195f0f)