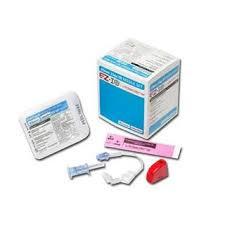
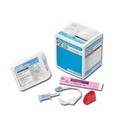
(EZ-IO) SET INTRA-OSSEOUS NEEDLE 15Gx25mm+ STABIL.DRESSING
Valid Article
INTRA-OSSEOUS INFUSION SYSTEM: EZ-IO Needle set
The intra-osseous needle set is worded and codified by single unit.
Definition
Battery powered medical drill and sterile medical devices needed to perform an intraosseous (IO) infusion (i.e., an infusion directly into the bone's medullary cavity) in children or adults.
Intraosseous infusion is used in emergency care when peripheral vascular acces is difficult, impossible or uneffective; especially for patients for whom immediate vascular access is lifesaving (e.g. patients with shock, severe dehydration, burns or cardiac / respiratory arrest)
Specifications
Technical specifications
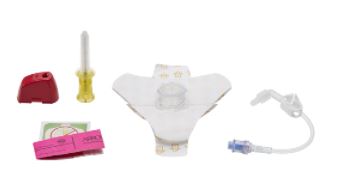
NEEDLE + STABILIZER SET
- 1 x EZ IO needle, 15G (Ø 1.8 mm)
- austenitic stainless steel needle (18/8)
- markings / 5 mm indicating depth, 5 mm of the catheter (at least one black line) must remain visible
- Luer lock connector
- with stylet for insertion and protective cap
- sterile, for single use
- length of the needle: :
- ref 9018P: 15 mm long, pink, for patients weighing between 3 to 39kg
- ref 9001P: 25 mm long, blue, for patients weighing more than 3kg
- ref 9079P: 45 mm long, yellow, for patients weighing more than 40 kg
- 1 x EZ-CONNECT extension set
- adapted to EZ IO PD needle
- needed to connect the IO needle safely to the infusion set
- with clamp
- sterile, for single use
- 1 x security wristband to document date and time of insertion
- 1 x ADHESIVE DRESSING
- sterile, single use
- size : 14.6 cm x 13.3 cm x 3.2 cm
- latex-free
- 1 x NeedleVISE 1 port: device for securing the needle after insertion, to be disposed of in a sharps container.
Packaging & Labelling
Sterile peel-open pack for every needle set, including instructions for use.
The sets are packed per 5
Instructions for use
Duration of use:
- For intraosseous access any time in which vascular access is difficult to obtain in emergent, urgent, or medically necessary cases, for up to 24 hours.
- For patients ≥12 years old, the device may be extended for up to 48 hours when alternative intravenous access is not available or reliably established.
Care & Maintenance
- Keep extremities secure to minimize movement.
- Assess frequently for extravasation, swelling, and optimal flow rate.
- Ensure site is securely in place, connections are secure, and dressing is clean, dry and intact.
- Confirm patency/placement prior to and throughout medication and fluid administration.
To remove EZ-IO:
- remove extension set and dressing
- attach Luer-lock syringe to the catheter hub and withdraw the catheter by applying traction while rotating clockwise. Do NOT rock or bend the catheter.
Precautions for Use
- Use moderate steady downward pressure and allow needle set to penetrate the bone, do NOT use excessive force!



![[KMEDMHHE17-] (mod hospital) INTRAOSSEOUS INFUSION KIT](/web/image/product.template/572593/image_256/%5BKMEDMHHE17-%5D%20%28mod%20hospital%29%20INTRAOSSEOUS%20INFUSION%20KIT?unique=94d2a0a)
![[KMEDMHAE221] (mod AMP) COMPLEMENTARY RESUSCITATION EQUIPMENT](/web/image/product.template/572665/image_256/%5BKMEDMHAE221%5D%20%28mod%20AMP%29%20COMPLEMENTARY%20RESUSCITATION%20EQUIPMENT?unique=96c00d0)
![[KMEDMHIS24-] (mod ICU) INJECTION SUPPLIES 2021](/web/image/product.template/574424/image_256/%5BKMEDMHIS24-%5D%20%28mod%20ICU%29%20INJECTION%20SUPPLIES%202021?unique=aecada5)
![[KMEDMHEE321A] (mod emergency) COMPLEMENTARY RESUSCITATION EQUIPMENT 2021](/web/image/product.template/574367/image_256/%5BKMEDMHEE321A%5D%20%28mod%20emergency%29%20COMPLEMENTARY%20RESUSCITATION%20EQUIPMENT%202021?unique=4c1ecd0)
![[KMEDMHOS33-] (mod OT Room) INJECTION SUPPLIES 2021](/web/image/product.template/574470/image_256/%5BKMEDMHOS33-%5D%20%28mod%20OT%20Room%29%20INJECTION%20SUPPLIES%202021?unique=e4f6df6)