TUBE, ENDOTRACHEAL, s.u. + CONN., microcuff , Ø 4 mm
Valid Article
BASIC ENDOTRACHEAL TUBE, microcuff
Definition
The endotracheal tube is a tube that is placed in the trachea through the mouth or nose and between the vocal cords. It serves to provide oxygen and inhaled gases to the lungs and protects the lungs from contamination, such as gastric contents or blood. It secures the airway and permits manual or mechanical ventilation in anaesthesia or resuscitation procedures.
Synonym
tracheal tube, Magill's tube
Specifications
Types of endotracheal tubes according to the specifications of the cuff
A cuff is an inflatable balloon at the distal end of the ETT. The inflated cuff produces a seal against the tracheal wall; this prevents gastric contents from entering the trachea and facilitates the execution of positive pressure ventilation.
The cuff inflates by attaching an appropriate size syringe to the pilot balloon. The syringe provides air under pressure and inflates both the pilot balloon and the cuff. Once the cuff inflates, the syringe needs to be removed, or the air in the cuff may redistribute back to the syringe and deflate the cuff. If the pilot balloon does not hold air, it must be assumed the cuff of the ETT has been damaged and is non-functional.
Cuffs are part of a cuff system consisting of the cuff itself plus a means of inflation, which includes a lumen in the wall of the tube, an external tube or inflating duct (portion that is visible outside the patient), a pilot balloon (indicating the inflation status of the cuff) and a non-return valve with Luer connection. Principal function of the cuff is to ensure a proper sealing and to center the ETT in the trachea.
- No cuff: distal end with Murphy eye (optional)
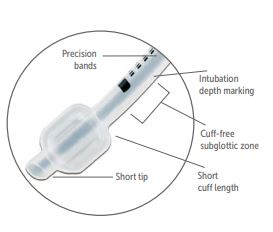
- Micro cuff: this tube has a short, ultra-thin PU cuff located away from the subglottic region; an important feature for children: it is the narrowest portion of the glottis and thus vulnerable to cuff-induced damage. The Murphy eye has been eliminated, which has allowed the cuff to be moved more distally on the ETT shaft. The cuff provides a seal with cuff pressure less than 10 cm H2O. This type of ET has a low tube exchange rate.
- Regular cuff: high volume, low pressure (HVLP) cuff with Murphy eye
Quality standards
Technical specifications
- Tube:
- preformed: the curvature of the ETT is called "the Magill Curve", it is a curvature of approximately 140 mm radius, +/- 20 mm.
- transparent PVC
- size marked on the tube (inner diameter in mm)
- graduated every cm
- open distal end with atraumatic 38º bevel, open on the left
- proximal end with standard connector, 15 mm outer Ø, for connecting to the ventilation system
- radio-opaque line to confirm correct placement
- Endotracheal cuff
- Location: near the distal end of the cannula, the end of the cuff must not iherniate over the tube tip under normal conditions; and the cuff must inflate symmetrically around the ETT.
- The cuff is inflated through a spring-loaded valve with a Luer lock connector. Attached to the valve is a pilot balloon which allows for (rough) tactile and visual confirmation of cuff inflation after intubation or deflation just before extubation.
- Material of the cuff wall: PU: microthin (10 µm) PU is used, the material is draping well over over the irregular thracheal mucosa contours.
- Low pressure cuff: average cuff pressure of +/- 10 cm H20
- Microcuff size: 10–12 mm for the sizes ID 3–5 mm
- Sterile, for single use
- Comes in different sizes: endotracheal tubes size refers to its internal diameter in millimeters. The ETT will typically list both the inner diameter (ID) and outer diameter (OD) on the tube. The narrower the tube, the greater resistance to gas flow.
Packaging & Labelling
Unit sterile packaging in peel-open pack
Instructions for use
Precautions for Use
Placing of an endotracheal tube should be done by skilled staff.
It requires the use of accessories (laryngoscope, Magill forceps...), as well as specific equipment for manual ventilation and tracheal suction.
There is enough clinical evidence available to proof that 'feeling' the pilot balloon does not give an accurate estimation of cuff inflation, particularly cuff over-inflation which can lead to tracheal mucosal ischemia and subsequent scarring, especially after longer-term intubation. It is therefore important to avoid high cuff pressures and check inflation with a cuff manometer.







![[EANECAME01-] CAPNOGRAPH, handheld, battery (Emma) mmHg](/web/image/product.template/575491/image_256/%5BEANECAME01-%5D%20CAPNOGRAPH%2C%20handheld%2C%20battery%20%28Emma%29%20mmHg?unique=9bc35c8)
![[EANEFOMA1P-] FORCEPS, MAGILL, paediatric, 15 cm](/web/image/product.template/569488/image_256/%5BEANEFOMA1P-%5D%20FORCEPS%2C%20MAGILL%2C%20paediatric%2C%2015%20cm?unique=8b9dd61)
![[KMEDMHIS25-] (mod ICU) CATHETERS, TUBES and DRAINS 2021](/web/image/product.template/574344/image_256/%5BKMEDMHIS25-%5D%20%28mod%20ICU%29%20CATHETERS%2C%20TUBES%20and%20DRAINS%202021?unique=aecada5)
![[KMEDMHES35-] (mod emergency) CATHETERS, TUBES & DRAINS 2021](/web/image/product.template/574355/image_256/%5BKMEDMHES35-%5D%20%28mod%20emergency%29%20CATHETERS%2C%20TUBES%20%26%20DRAINS%202021?unique=4c1ecd0)
![[KMEDMHOS45-] (mod OT Room) CATHETERS AND DRAINS 2021](/web/image/product.template/574392/image_256/%5BKMEDMHOS45-%5D%20%28mod%20OT%20Room%29%20CATHETERS%20AND%20DRAINS%202021?unique=6abd431)
![[KMEDMHAS15-] (mod AMP) CATHETERS, TUBES & DRAINS](/web/image/product.template/572672/image_256/%5BKMEDMHAS15-%5D%20%28mod%20AMP%29%20CATHETERS%2C%20TUBES%20%26%20DRAINS?unique=9a70d84)