RACHI SET NRFit, Whitacre 25Gx90mm, 5ml Lock syringe + acc1
Valid Article
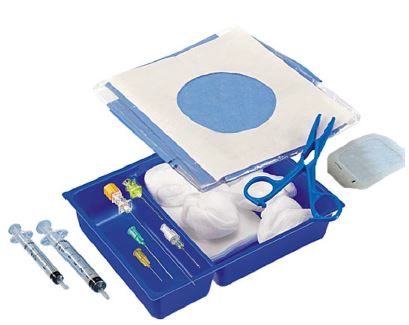
RACHI SET NRFit
Definition
A collection of devices intended for the administration of an anaesthetic agent (not included) in the cerebrospinal fluid for the management of pain during surgery. It includes a spinal needle and additional procedural devices (e.g., syringe, wipes). The different injection devices have a NRFit connection to avoid errors of connections with Luer devices. This is a single-use device.
Synonym
spinal set, spinal tray
Specifications
Quality standards
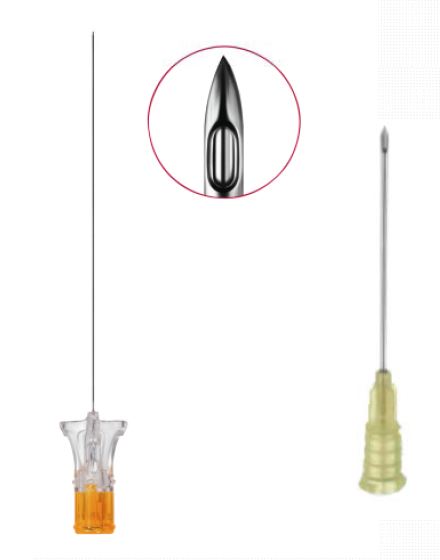
- ISO 80369-6, 2025, edition 2, Small bore connectors for liquids and gases in healthcare applications Part 6: Connectors for neuraxial applications
- ISO 80369-1, 2018, edition 2, Small-bore connectors for liquids and gases in healthcare applications - Part 1: General requirements
Quality Standards Comment
ISO 80369-6:2016 specifies requirements for small-bore connectors intended to be used for connections in neuraxial applications. Neuraxial applications involve the use of medical devices intended to administer medications to neuraxial sites, wound infiltration anaesthesia delivery, and other regional anaesthesia procedures or to monitor or remove cerebro-spinal fluid for therapeutic or diagnostic purposes.
NOTE 1 Sites for the neuraxial application include the spine, intrathecal or subarachnoid space, ventricles of the brain, and the epi-, extra-, or peri-dural space. Neuraxial application anaesthetics can be administered regionally affecting a large part of the body, such as a limb, and include plexus blocks, such as the branchial plexus blocks or single nerve blocks. Neuraxial application procedures include continuous infusion of wounds with local anaesthetic agents.
Technical specifications
- double tray set
- co-package of the procedure set with the spinal needle (in its primary package) on top of it.
- sterile, for single use
- qty = 1: NEEDLE SPINAL ANAESTH. NRFit, pencil point, 25G x 90mm, with guide: the spinal needle is placed (in its primary package) on top of the procedure pack, inside the co-package. All sets will contain the 25G/90mm needle, in case another needle is necessary it will have to be taken from the stock
- qty = 1: DRAPE, non-woven, fenestrated 7x12cm, adhesive transparent borders, 75x95 cm, on top of the 2 trays
- qty = 1: DRAPE, non-woven, 75x90cm, used as outer wrap of the 2 trays + the fenestrated drape, can be used as sterile drape for the table.
- qty = 3: Applicator sponge, small, in the top tray
- qty = 2: GALLIPOT, 50 - 100 ml, s.u, for NaCl and disinfectant, one red and one transparent, in the top tray
- qty = 5: Gauze compresses, 10 cm, 8 ply: in the bottom tray
- qty = 1: NRFit blunt fill needle, 18G, filter needle: in the bottom tray
- qty = 1: SYRINGE NRFit-Lock, 5 ml, s.u.: in the bottom tray together with the fill needle
MSF requirements
With the introduction of the NRFit connector we will move at the same time to the use of a spinal anesthesia set (without medications = avoid importation / sourcing issues). The set is to be used for each spinal anesthesia, only one set is proposed with a 25G needle (technique of choice). In case another needle is preferred, this needle must be used in combination with the set.



![[SINSSYNRL03] SYRINGE, s.u., NRFit Lock, 3 ml](/web/image/product.template/587639/image_256/%5BSINSSYNRL03%5D%20SYRINGE%2C%20s.u.%2C%20NRFit%20Lock%2C%203%20ml?unique=a6dae49)
![[SINSSYNRL05] SYRINGE, s.u., NRFit Lock, 5 ml](/web/image/product.template/587638/image_256/%5BSINSSYNRL05%5D%20SYRINGE%2C%20s.u.%2C%20NRFit%20Lock%2C%205%20ml?unique=a6dae49)
![[SINSTRNR01-] FILL NEEDLE NRFit, 18G, blunt, 5 µm filter, sterile, s.u.](/web/image/product.template/587640/image_256/%5BSINSTRNR01-%5D%20FILL%20NEEDLE%20NRFit%2C%2018G%2C%20blunt%2C%205%20%C2%B5m%20filter%2C%20sterile%2C%20s.u.?unique=a6dae49)