ARTERIAL ACCES SET, femoral 18Gx12cm, placement set, sterile
STD
SINSCACA1812S
Valid Article
HS Code:
901890
Last Updated on:
09/08/2025, 22:02:06
Single use
A single-use device, also referred to as a disposable device, is intended for use on one patient during a single procedure. It is not intended to be reprocessed (i.e., cleaned and disinfected or sterilized).
CE marking: declaration that the product meets EU standards for health, safety, and environmental protection. The CE marking indicates that the product may be sold freely in any part of the European Economic Area, regardless of its country of origin.
C0504 - Arterial introduction sets
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes
Thermosensitive codes are defined for storage and transportation temperature requirements of the products.
CENTRAL ARTERIAL CATHETERIZATION SET
Definition
Catheter for arterial access intended for invasive blood pressure monitoring an obtaining blood samples for arterial blood gases analysis. The set contains also devices needed for the placement of the arterial catheter using the Seldinger technique.
Specifications
Some restricted information has been hidden. Sign in
to see this information
Quality standards
EN ISO 10555-1, 2013, edition 3, +A1 2017 Sterile, single-use intravascular catheters - Part 1: General requirements
Technical specifications
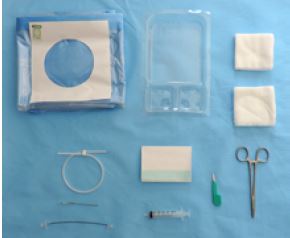
- Femoral set 5118.212
- 1 arterial catheter 18 G - 12 cm Transparent PTFE (polytetrafluoroethylene) catheter tube, with 2 black O.R.X lines with a winged base to facilitate catheter attachment
- 1 introducer needle 19G x 52 mm
- 1 peelable application surface 100 x 150 cm
- 10 non-woven compresses 7.5 x 7.5 cm
- 1 catheter dressing 14 x 10 cm
- 1 syringe 5 ml Luer Slip
- 1 needle holder 14 cm
- 1 scalpel N°11 short sleeve
- 1 tray with 3 compartments 19 x 13 x 3 cm
- Radial set 5118.906
- 1 arterial catheter 20 G - 6 cm Transparent PTFE (polytetrafluoroethylene) catheter tube, with 2 black O.R.X lines with a winged base to facilitate catheter attachment
- 1 introducer needle 20G x 35 mm
- 1 peelable application field 50 x 97 cm 5.5 x 7.5 cm adhesive window
- 10 non-woven compresses 7.5 x 7.5 cm
- 1 catheter dressing 14 x 10 cm
- 1 syringe 5 ml Luer Slip
- 1 needle holder 14 cm
- 1 scalpel N°11 short sleeve
- 1 tray with 3 compartments 19 x 13 x 3 cm
- Sterile, for single use
Packaging & Labelling
Sterile set in a tray peel open pack.
MSF requirements
Article needed for continuous invasive blood pressure monitoring for unstable intubated patients + easy blood sampling for repeated arterial blood gases analysis :
- Mechanical ventilation for acute respiratory failure
- And/or hemodynamic failure
Some restricted information has been hidden. Sign in
to see this information
Some restricted information has been hidden. Sign in
to see this information