OROPHARYNGEAL AIRWAY, s.u., non ster., 80mm, ID 4.5mm, green
Outdated Article
OROPHARYNGEAL AIRWAY, disposable
Definition
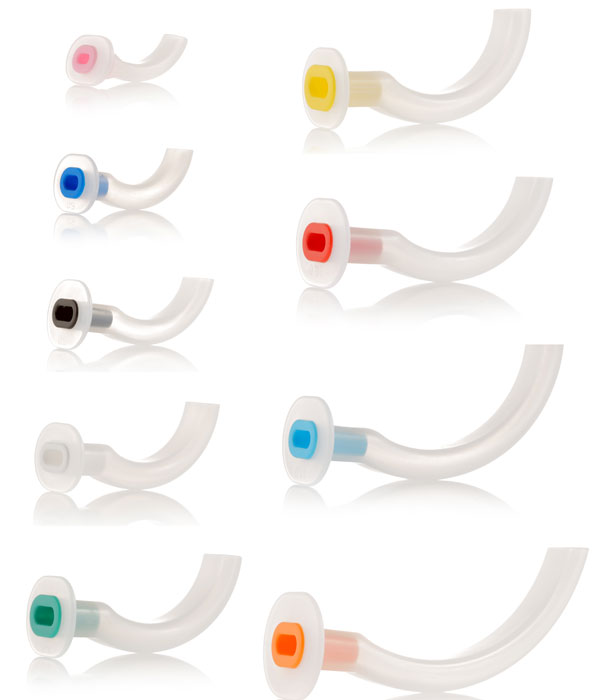
A curved plastic tube inserted through the mouth to facilitate airway patency for gas exchange or suctioning. The device prevents the tongue from obstructing airflow. This rigid tube offers a bite-resistant passage for air from the lips to the posterior pharynx. This is a single-use device.
Synonym
oral airway, OPA, Guedel airway, Mayo cannula
Specifications
Quality standards
- ISO 5364, 2016, edition 5, (confirmed 2022) Anaesthetic and respiratory equipment - Oropharyngeal airways
- ISO 18190, 2016, edition 1, Anaesthetic and respiratory equipment - General requirements for airways and related equipment
Technical specifications
- Design: Guedel
- Material: plastic, latex-free
- Parts:
- curved and flattened distal (or pharyngeal) end
- oval aperture, same lumen from proximal to distal end
- Straight and reinforced proximal (or buccal) end = bite block
- proximal flange (collar)
- indicates the size by number and color code
- is intended to be external to the teeth or gums
- Non sterile, for single use
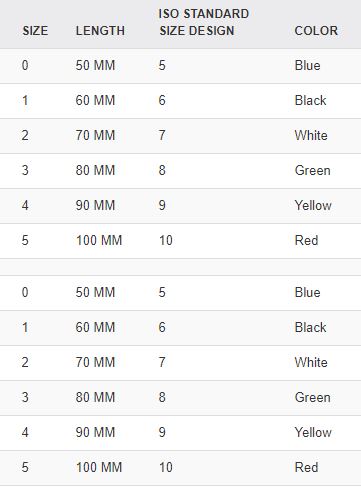
Different sizes: the size is identified on the flange of the cannula (ISO size = length in cm) and with the standard colour as defined by the ISO.
Code | Use | Size ISO | Length | Internal diameter | Colour |
SCTDAIRGN040 | Neonate | 4 | 40 +/- 2.5 mm | 3 mm | Pink |
SCTDAIRGN050 | Baby | 5 | 50 +/- 2.5 mm | 3.5 mm | Blue |
SCTDAIRGN060 | Small child | 6 | 60 +/- 2.5 mm | 4.0 mm | Black |
SCTDAIRGN070 | Child | 7 | 70 +5.0 / -2.5 mm | 4.0 mm | White |
SCTDAIRGN080 | Adolescent | 8 | 80 +/- 5.0 mm | 4.5 mm | Green |
SCTDAIRGN090 | Adult | 9 | 90 +/- 5.0 mm | 4.5 mm | Yellow |
SCTDAIRGN100 | Large adult | 10 | 100 +/- 5.0 mm | 5.0 mm | Red |
Packaging & Labelling
Quantity per box differs according the manufacturer.
Instructions for use
The proper size is selected by holding the airway against the side of the patient's face and ensuring that it extends from the corner of the mouth to the angle of the jaw. If the airway is improperly sized, it will occlude the airway.
An oral airway is placed by inserting a tongue depressor into the patient's mouth to displace the tongue downward and then passing the airway into the patient's mouth, slipping it over the patient's tongue.
In adults it is inserted into the mouth upside-down, and rotated into the normal position once it is inserted about half way.
Lubricant may be useful if the mouth is excessively dry.
MSF requirements
Replaces the reusable cannulas as these become rare in the market.
The sterility of the device is not a requirement, but is an option that may be choosen because of availability. In this case the specific NST codes should be used to allow a proper stock management.





![[SCTDAIRG080S] OROPHARYNGEAL AIRWAY, s.u., sterile, 80mm ID 4.5mm green](/web/image/product.template/587599/image_256/%5BSCTDAIRG080S%5D%20OROPHARYNGEAL%20AIRWAY%2C%20s.u.%2C%20sterile%2C%20%2080mm%20ID%204.5mm%20green?unique=7311cb8)