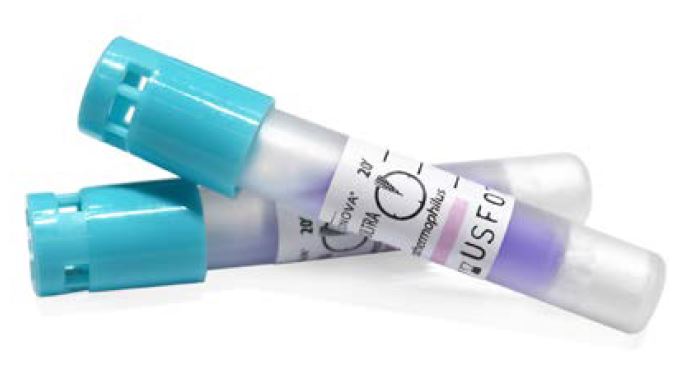
BIOLOGICAL INDICATOR, class B steam, BT224
Valid Article
BIOLOGICAL INDICATOR, class B steam BT224
Definition
A sterilization indicator consisting of a standardized, viable population of microorganisms (e.g., bacterial spores known to be resistant to the mode of sterilization being monitored) that will display a change when exposed to an effective sterilization outcome indicating that sterilization parameters have been met. This is a single-use device.
Specifications
Biological indicators, or spore tests, are the most accepted means of monitoring sterilization because they assess the sterilization process directly by killing known highly resistant microorganisms (e.g., Geobacillus or Bacillus species).
Quality standards
- ISO 11138-1, 2017, edition 3, (confirmed 2022) Sterilization of health care products — Biological indicators — Part 1: General requirements
- ISO 11138-3, 2017, edition 3, (confirmed 2022) Sterilization of health care products — Biological indicators Part 3: Biological indicators for moist heat sterilization processes
Quality Standards Comment
USP 1035: Biological Indicators for sterilization
Technical specifications
- Biological Indicator System: Super Rapid: result in 20 minutes
- Plastic tube
- polypropylene
- height : 50.4 mm
- external diameter: 8.5 mm
- Wall thickness: 0.5 mm
- Cap
- colour: light blue
- external diameter: 10.7 mm
- height: 16.4 mm
- Cap filter:
- medical grade paper
- 60g/m2
- diameter: 17 mm
- Glass ampoule
- height: 35.0 - 40.0 mm
- external diameter: 6.8 mm
- wall thickness: 0.2 – 0.3 mm.
- Culture medium:
- 0.5-0.7 ml
- purple colour
- Spores carrier: Filter Paper: 5.0 x 25.0 mm, 160 g/m2
- ≥ 106 Geobacillus stearothermophilus ATCC 7953 spores per vial
- Incubation Period : 20 Minutes
- Sterilization Method: Steam
- Dynamic-air-removal (vacuum assisted steam sterilizer):
- 134˚C (275˚F) 10 min.
To be Ordered Separately
- ESTEBIRE02- BIOLOGICAL INDICATOR READER / INCUBATOR (MiniBio)
- ESTETBOD1-- TEST, BOWIE-DICK, ready to use pack, s.u.
- ESTETSTI51- TST INDICATOR STRIP, Type 5
Instructions for use
Product Use: Load Monitoring
Use the Biological Indicator BT224 in conjunction with both the MiniBio Auto-Reader incubatorto qualify or monitor dynamic-air-removal steam sterilization cycles of 10 minutes at 134°C (174 °F)
- Final fluorescence reading is performed after 20 minute-incubation at 60 ºC (sensitivity ≥ 97 %).
- An optional visual pH color change confirmation could be made after 48 hours of incubation. If sterilization process has not been successful, culture medium will change to a greenish color first, and then to yellow during incubation at 60 ºC, thus showing the presence of living spores. If sterilization process is successful culture medium will remain purple after the incubation process.
- 7-day readout is optional and not intended to be routinely performed; it is an initial validation of the 20 minute-reading. Fluorescence results may be compared to the 7-day visual reading.
- NOTE: if 7-day readout is performed, a humidified environment will be required to avoid medium to dry out.
- D-Value: Not lower than 1.5 minutes at 121 ºC. Another D-value is informed at 132ºC y a 135ºC.
- Z-Value: Not lower than 6 ºC.
Frequency:
- Should be used for routine monitoring of steam sterilization cycles at least weekly, preferably daily, each time the autoclave is used.
- In every load containing implants (All loads that contain implantable devices - that are implanted in the Operating Theatre and remain in the patient for at least 30 days).
Precautions for Use
- Do not store the product near sterilizing agents.
- Do not expose this product to Ethylene Oxide, Dry Heat, Radiation or any sterilization process other than Steam.
Storage
- Temperature between 10-30 ºC
- Relative Humidity 30-80 %
- Keep in a dark place in its original box
- Shelf life: 24 months
MSF requirements
Included as part of the Quality Assurance test for the new Tuttnauer autoclave Class B.
Are required:
- ESTEBIRE02- BIOLOGICAL INDICATOR READER / INCUBATOR (MiniBio)
- ESTEBIRT0201 BIOLOGICAL INDICATOR, steam, air-remov., BT224
- ESTETBOD1-- TEST, BOWIE-DICK, ready to use pack, s.u.
- ESTETSTI51- TST INDICATOR STRIP, Type 5
The sterilization procedure should be monitored routinely by using a combination of mechanical, chemical, and biological indicators (BIs) to evaluate the sterilizing conditions and indirectly the microbiologic status of the processed items.
BIs measure the sterilization process directly by using the most resistant microorganisms (i.e., Bacillus spores); they are designed to demonstrate whether the conditions during a steam (autoclave) cycle were adequate to achieve a defined level of microbial inactivation.







![[ESTEBIRE02-] BIOLOGICAL INDICATOR READER / INCUBATOR (MiniBio)](/web/image/product.template/587588/image_256/%5BESTEBIRE02-%5D%20BIOLOGICAL%20INDICATOR%20READER%20-%20INCUBATOR%20%28MiniBio%29?unique=481c0f1)