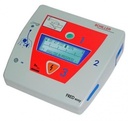
SEMI-AUTOMATED EXTERNAL DEFIBRILLATOR (FRED easy), manual Fr
Outdated Article
EXTERNAL DEFIBRILLATOR (FRED easy)
The article has been stopped in 2022 because of supply issues, and the fact that the lithium batteries are non rechargeable.
It is replaced by the new defribrillator AED PRO from Zoll (see “replaced by” at bottom of the page).
Definition
A portable electronic device designed to automatically detect cardiac arrhythmias (ventricular fibrillation/pulseless ventricular tachycardia) in a sudden cardiac arrest (SCA) patient, after which it audibly/visually instructs an operator to enable it to activate defibrillation of the heart through application of electrical shocks to the chest surface. The device is intended to be operated by a layperson. It consists of an external pulse generator (EPG) and a pair of skin-adhesive electrodes to monitor the rhythm and deliver the shocks.
Specifications
Quality standards
Technical specifications
- Semi-automatic defibrillation for adults or children:
- adults: biphasic energy 90-130-150 J
- children (automatic switch when paediatric electrodes are plugged in): 15-30-50 J
- Automatic charge control if shock is recommended based on the analysis
- Charging time < 10 seconds
- LCD display, high resolution, with background lighting:
- passed time
- number of delivered shocks
- battery and memory capacity
- electrode type
- test instructions
- Electrode connection indicator
- LED flashing light for device ready and service status (including battery status)
- Speaker for spoken instructions (adjustable volume)
- Lithium battery operated: LiMnO2 12V 2.8Ah (> 2g)
- Battery capacity:
- 180 shocks
- 7 hours operation
- 5 years in stand-by (with internal self-test of battery)
- Protection against electric shock
- Type: BF
- Protection against solid/ liquid ingress: IPx4
- Dimensions: 70 x 230 x 220 mm
Transport Dangerous Goods
- UN3091 Lithium metal batteries packed with equipment
- Class: 9
- Packing instructions: 969 Section I
- Limit per package:
- Pax A/C (Passengers & Cargo Aircraft) = 5 kg
- CAO (Cargo Aircraft Only) = 35 kg
Supplied with the Article
- 5 pairs of adult adhesive electrodes
- 2 pairs of paediatric adhesive electrodes
- 1 lithium battery (LiMnO2)
- 1 carrying bag
- 1 SD memory card
- 1 instructions manual
Instructions for use
Equipment intended for the management of cardiac arrests by medical or paramedical staff who is trained in this technique. Reserved for emergency room, operating theatre or intensive care unit.
Read the instructions manual carefully.
On-screen and verbal instructions prompts to support user through all the process (English or French mode depending on the selected configuration).
Precautions for Use
Follow the usual safety rules related to the use of a defibrillator (no interference between patient and staff during defibrillation, etc.)
MSF requirements
Semi-automatic device that can be used in automatic mode by paramedical staff or according to the ECG analysis by qualified physicians.




![[EEMDDEFA104] (defibrillator FRED easy) WALL MOUNTED CASE](/web/image/product.template/570906/image_256/%5BEEMDDEFA104%5D%20%28defibrillator%20FRED%20easy%29%20WALL%20MOUNTED%20CASE?unique=f4aa7ae)
![[EEMDDEFC101] (defibrillator FRED easy) ELECTRODE, adhesive, adult, pair](/web/image/product.template/570907/image_256/%5BEEMDDEFC101%5D%20%28defibrillator%20FRED%20easy%29%20ELECTRODE%2C%20adhesive%2C%20adult%2C%20pair?unique=47fbbba)
![[EEMDDEFC102] (defibrillator FRED easy) ELECTRODE, adhesive, child, pair](/web/image/product.template/570901/image_256/%5BEEMDDEFC102%5D%20%28defibrillator%20FRED%20easy%29%20ELECTRODE%2C%20adhesive%2C%20child%2C%20pair?unique=47fbbba)
![[EEMDDEFE9F-] SEMI-AUTOMATED EXTERNAL DEFIBRILLATOR (AED Pro), manual, fr.](/web/image/product.template/577963/image_256/%5BEEMDDEFE9F-%5D%20SEMI-AUTOMATED%20EXTERNAL%20DEFIBRILLATOR%20%28AED%20Pro%29%2C%20manual%2C%20fr.?unique=116a2f7)
![[EEMDDEFS103] (defibrillator FRED easy) BATTERY LiMnO2 non rech. 4-07-001](/web/image/product.template/570902/image_256/%5BEEMDDEFS103%5D%20%28defibrillator%20FRED%20easy%29%20BATTERY%20LiMnO2%20non%20rech.%204-07-001?unique=25149f4)