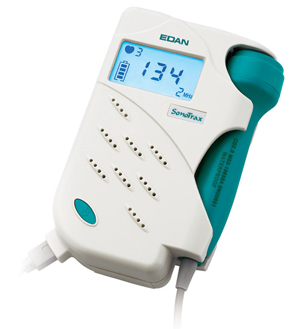
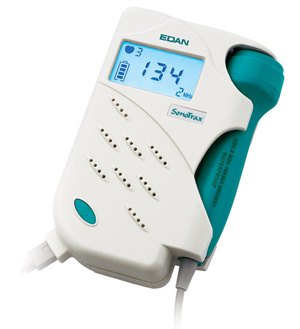
FOETAL DOPPLER SYSTEM, ultrasonic (Sonotrax)
Valid Article
FOETAL DOPPLER SYSTEM
Definition
Device allowing to hear and to display the fetal heart rate from 10-12 weeks gestation.
This fetal heartbeat detector works on the principle of DOPPLER effect. Ultrasonic waves are beamed from the transducer probe to the foetus. The beam is reflected back with slight difference in frequency due to fetal heart movements, blood particles or other moving organs. The reflected signal is received, amplified and electronically processed by the device to produce audible beeps.
Synonym
Doppler, Foetal heart detector
Specifications
Technical specifications
- Ultrasound frequency: 2MHz (obstetrical probe)
- Hearing of fetal heartbeat:
- Loudspeaker with volume control
- Output power: 0.5 W
- Recording length: 240 s
- Calculation of the fetal heart rate:
- Range: 50-210 bpm (beats per minute)
- Accuracy: < ± 1 bpm over 100-180 bpm
- Averaging: 3 – 5 beats
- LCD display (digital screen) of the fetal heart rate
- Waterproof probe and cable
- Power supply:
- Runs on 2 AA batteries of 1.5 V
- Battery life: 8 hours
- Charge time: 4 hours
- "Battery low" signal: only on basic A model
- Auto power-off (after 1 min)
Dimensions
- Main unit: 144 x 89 x 33 mm
- Probe: 88 mm x Ø 35 mm
- Total weight: 300 g (including batteries)
Packaging & Labelling
Supplied in a soft pouch with:
- 2 AA rechargeable batteries
- 1 battery charger
- 1 tube of clear ultrasound gel
- 1 user's guide
Instructions for use
In case of skin infection, cover the end of the probe with a condom or a single use latex glove.
If ultrasound gel is difficult to get, it can be replaced by olive oil or soft almond oil.
Maintenance
The probe cannot be sterilized and must be cleaned and disinfected with a detergent/disinfectant for surfaces/non-invasive medical equipment. Prepare use-solution in concentration required. Always read the label and product information before use. Do not mix with other products. Make sure to wet the surfaces completely, use a wipe or towel, keep them wet for the whole exposure time.
(Cf Introduction: Disinfection and sterilization in the field)
Storage
Protected from humidity and dust.
MSF requirements
Easy to use, this device is useful for the diagnoses of fetal distress, fetal death, molar pregnancy and multiple pregnancy.

![[EDIMULSC1CA] (Ultrasound) COUPLING AGENT, 250 ml bot.](/web/image/product.template/570107/image_256/%5BEDIMULSC1CA%5D%20%28Ultrasound%29%20COUPLING%20AGENT%2C%20250%20ml%20bot.?unique=998a964)
![[EEMDFHDE302] (Sonotrax basic) MAIN UNIT FOETAL HEART DETECTOR w/o probe](/web/image/product.template/570775/image_256/%5BEEMDFHDE302%5D%20%28Sonotrax%20basic%29%20MAIN%20UNIT%20FOETAL%20HEART%20DETECTOR%20w-o%20probe?unique=b112249)
![[EEMDFHDA301] (doppler Sonotrax) PROBE 2 MHz, waterproof MS3-14320](/web/image/product.template/570777/image_256/%5BEEMDFHDA301%5D%20%28doppler%20Sonotrax%29%20PROBE%202%20MHz%2C%20waterproof%20MS3-14320?unique=c53c3c2)
![[EEMDFHDA303] (Sonotrax basic) PROBE 8MHz, waterproof, 606260](/web/image/product.template/568548/image_256/%5BEEMDFHDA303%5D%20%28Sonotrax%20basic%29%20PROBE%208MHz%2C%20waterproof%2C%20606260?unique=af2b278)
![[EEMDFHDS301] (Sonotrax basic) HOUSING UPPER AND LOWER, 702677](/web/image/product.template/581549/image_256/%5BEEMDFHDS301%5D%20%28Sonotrax%20basic%29%20HOUSING%20UPPER%20AND%20LOWER%2C%20702677?unique=a048a29)
![[KMEDMHCE19A] (mod OPD) GYNAECO/OBSTETRIC MATERIAL VERSION A](/web/image/product.template/572675/image_256/%5BKMEDMHCE19A%5D%20%28mod%20OPD%29%20GYNAECO-OBSTETRIC%20MATERIAL%20VERSION%20A?unique=5ddfa52)
![[KMEDMHHE19B] (mod hospital) GYNAECO/OBSTETRIC MATERIAL VERSION B](/web/image/product.template/572757/image_256/%5BKMEDMHHE19B%5D%20%28mod%20hospital%29%20GYNAECO-OBSTETRIC%20MATERIAL%20VERSION%20B?unique=b897390)