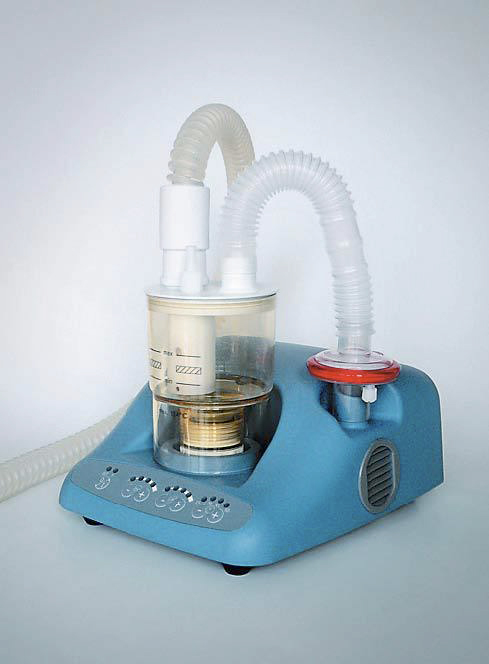
ULTRASONIC NEBULIZER (Ultraneb) + accessories
Outdated Article
ULTRASONIC NEBULIZER
Accessories, consumables and spare-parts are no longer available from the manufacturer in 2022.
Stopped in June 2018 and becomes NST in consultation of the TB working group (Gabriella Ferlazzo). The ultrasound nebulizer can be replaced by the Nebulizer + compressor PARI Boy Pro EEMDNECE6-- (see “replaced by” at bottom of the page).
Definition
Used with a medication cup this device generates aerosolised medication (finely dispersed airborne droplets) for inhalation by a patient with respiratory disorders.
Without a medication cup, the device can be used for induction in order to obtain sputum samples necessary for the diagnosis of tuberculosis.
It consists of a mains electricity (AC-powered) electronic oscillator, an ultrasound transducer (piezoelectric crystal), a coupling basin, a nebulizer chamber and a fan. A high frequency (e.g., 1-2 MHz) electrical current is applied to the crystal within the oscillator circuit converting it to mechanical vibrations which produce sound waves of 1 to 2 MHz. The coupler (water or saline) transmits the sound waves to the (medicated) solution in the nebulizing chamber.
Specifications
Quality standards
Quality Standards Comment
- EN 13544-1+A1: 2009 "Respiratory therapy equipment - Part 1: Nebulizing systems and their components"
Material
The material of the nebulization chamber and the patient circuit allow a sterilization at 134°C, except the quartz that should be removed before sterilization!
Components
Ultrasonic nebulizer including:
- 48 x single use mouth piece (ref. 49-2002-02-1-306)
- 4 x autoclavable hose of 300 mm (ref. 49-2002-02-1-146)
- 4 x autoclavable hose of 1200 mm (ref. 49-2002-02-1-147)
- 26 x antibacterial filter (ref. 49-1001005879)
- 1 x nebulization chamber (ref. 49-2002-02-1-550)
- 96 x single patient medication cups (ref. 100HD648)
- 70 x adult mask (ref. ? )
- 50 x paediatric mask (ref. ? )
- 2 x fuses T800mA 250V (ref. 33-FU.0.80AF)
Technical specifications
Ultrasonic nebulizer, desk model with easy to use settings:
- Adjustable timer function: 0, 15, 30, 45 and 60 min.
- Air-flow output (ml/min): 4 adjustable settings
- Adjustable nebuliser rate (quantity of particles in the flow)
- Separated air path for patient and device cooling
- Alarm settings:
- no nebuliser chamber attached
- low liquid level / empty chamber
- medication time completed
- device failure
Ambient conditions
- When using:
- temperature 10° to 40° C
- humidity 0 to 90% relative humidity non condensing
- During storage:
- temperature -20° a 70° C
- humidity 0 to 90% relative humidity non condensing
Performance data
- Power supply: 110V/230V ; 50/60 Hz
- Fuses: 800mA 250V, 2 pieces
- Power consumption: 50 VA
- Ultrasonic frequency: 1.68 MHz
- Sound level: 35 dB
- Humidity protection type IP 32 (dripping water)
Functioning method: continuous use
- Nebuliser rate: 3 ml/min
- Particle size (MMAD) average 4 μm, about 86 % inferior to 5 μm
- Airflow rate up to 20 l/min
Dimensions
- H x W x D: 19,0 x 20,5 x 31,5 cm
- Weight: 3.5 kg
Instructions for use
Read carefully instructions manual before use.
It is recommended to follow the manufacturer's instructions for use and maintenance of the device.
Precautions for Use
Contact your biomedical officer in order to see the need to protect the apparatus with a double conversion UPS (see related articles below).
Maintenance
- The anti-bacterial filter is for single use it should be replaced between each patient and at least every week if used with the same patient.
- All other parts must be sterilized by steam autoclave. CAUTION: remove the quartz module before!
- Mind not to scratch the piezoelectric crystal when cleaning.
- The machine should be dismantled, cleaned and disinfected after each patient with 0.5% detergent-disinfectant solution for medical equipment (i.e. 25 ml = 1 stroke of dosing pump for 5 litres water).
(Cf Introduction: Disinfection and sterilization in the field)
MSF requirements
Portable, easy to use.




![[EEMDNECE3--] NEBULIZER + COMPRESSOR (PARI BOY SX) + accessoires](/web/image/product.template/571017/image_256/%5BEEMDNECE3--%5D%20NEBULIZER%20%2B%20COMPRESSOR%20%28PARI%20BOY%20SX%29%20%2B%20accessoires?unique=f3eb687)
![[SCTDBRCF1A-] FILTER, BREATHING CIRCUIT, 22M/15F, adult/child, s.u.](/web/image/product.template/571898/image_256/%5BSCTDBRCF1A-%5D%20FILTER%2C%20BREATHING%20CIRCUIT%2C%2022M-15F%2C%20%20adult-child%2C%20s.u.?unique=6fb9a30)
![[SSCOSODC6V-] SODIUM chloride 6%, for nebulizer, 4 ml, vial](/web/image/product.template/572936/image_256/%5BSSCOSODC6V-%5D%20SODIUM%20chloride%206%25%2C%20for%20nebulizer%2C%204%20ml%2C%20vial?unique=1b5f9a6)