SWAB, cotton tip, wooden stick, 15 cm, ste, s.u.
STD
STSSSWAB15CWS
Valid Article
Account code:
60200
HS Code:
841990
Last Updated on:
09/05/2024, 22:05:52
Former
Code(s):
ELAESWAB1C- ELABSWAB1C- ELABSWAB15CWS ELAEMISC905 ELAEZBE0905
Single use
A single-use device, also referred to as a disposable device, is intended for use on one patient during a single procedure. It is not intended to be reprocessed (i.e., cleaned and disinfected or sterilized).
A1101 - Sample collection neutral swabs
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes
Thermosensitive codes are defined for storage and transportation temperature requirements of the products.
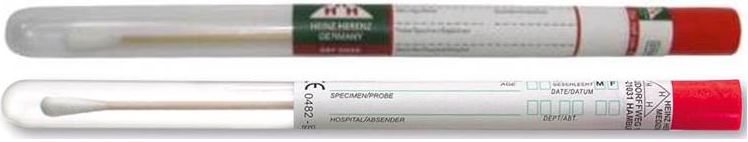
SWAB, wooden stick, sterile
Definition
Sterile dry swab used to take samples, contained in labelled a cylindrical tube.
Specifications
Technical specifications
- Plastic tube (PP) with label Ø 12 x 155 mm
- Wooden stick with cotton bud, ± 16 x 150 mm, fixed to the cap of the tube
- Sterile, for single use
Packaging & Labelling
Unit sterile packaging. Box of 100.
Instructions for use
Please consult the “Updated laboratory procedures, 2022” or “Bacteriology laboratory procedures and resources“ available online via the Laboratory working Group sharepoint page: Laboratory Procedures and Resources.
https://msfintl.sharepoint.com/sites/msfintlcommunities/LabWG/SitePages/Home.aspx
For offline access, contact your laboratory advisor.
Storage
- Store between 2 and 25°C
- Shelf life: 36 months
MSF requirements
Swabs are sterile and ready-for-use systems intended for collected clinical samples to be sent for cultural examination.
Swabs for general purpose: suitable for throat, vagina, skin.
Caution: Swabs with wooden sticks are not suitable for PCR analysis.
Some restricted information has been hidden. Sign in
to see this information