ISOLATION GOWN, s.u., non sterile
Valid Article
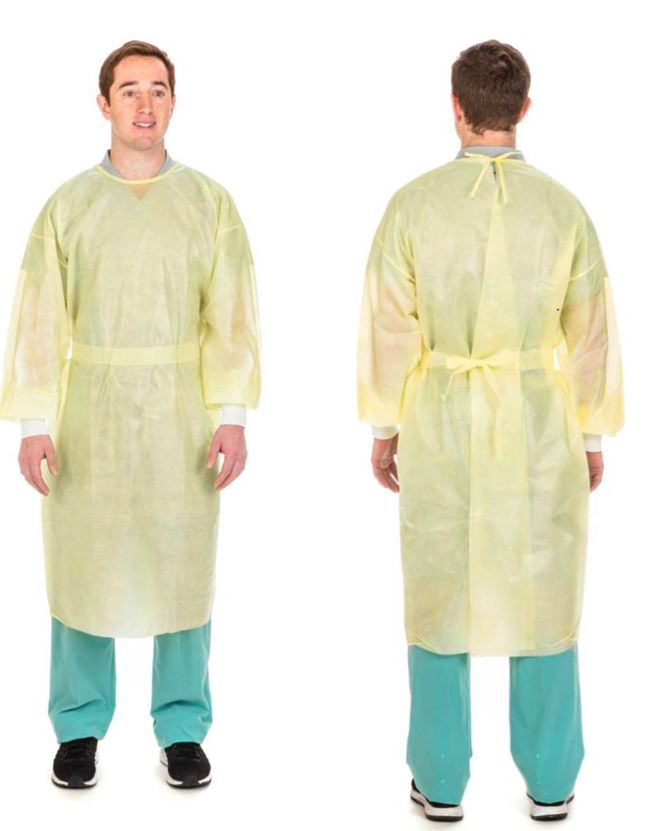
ISOLATION GOWN, single use
Definition
Non sterile single use garment intended to be worn by healthcare providers or visitors to protect the patient from the transfer of infectious agents. It may also help to protect the healthcare provider or visitor from a contagious agent which has infected the patient.
This gown has a limited liquid barrier protection but is not tested for infectious barrier properties.
Specifications
Quality standards
Technical specifications
The isolation gown ideally should not restrict the movement of the body and should be breathable and comfortable to wear for long periods.
Non-woven materials alone or in combination with materials that offer increased protection from liquid penetration: SMS, SMMS or polyethylene-coated polypropylene, or Chlorinated polyethylene elastomers (CPE) or equivalent
- non sterile
- water resistant: HH rate min 200 mm (ANSI / AAMI level2)
- breathable, flexible
- full back gown, closure in the back
- neck closure: 3 options
- velcro closure (hook and loop) is preferred
- tape tab neck closure
- tie closure
- waist tie that binds at the back or at the side (not in the front), the back has to be fully covered
- long sleeves
- cuffs at the wrists: 3 different types
- cotton or cotton / polyester blend knit cuff
- elastic around the wrist
- thumb loop (last choice as it complicates hand hygiene)
- single use
- Universal size, but coverage of the whole upper body till under the knees required
- length (measured at front from middle of neckline to bottom): 130 –145 cm
- circumference (measured at waist): minimum 130 cm
Instructions for use
To be worn when there is:
- a protection isolation of an immune-depressed patient, like burn patient, malnourished child, AIDS patient...
- an infectious isolation: contagious diseases transmitting by airborne contact (droplets)
Follow the rules of undressing and dressing.
Precautions for Use
Ties on the abdomen or torso, if present, should be tied properly: they may cause other hazards if not tied properly or not tied at all.
In order to eliminate the strike-through through the cuffs, gloving over the cuff is strictly recommended.




![[KMEDMHMI22-] MODULE, VHF INVESTIGATION 2 persons/10 samples 2021](/web/image/product.template/574349/image_256/%5BKMEDMHMI22-%5D%20MODULE%2C%20VHF%20INVESTIGATION%202%20persons-10%20samples%202021?unique=d3aabdf)