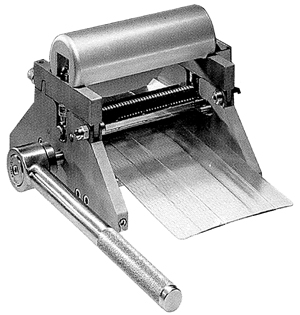
SKIN GRAFT MESHER, ZIMMER, ratio 1 to 3 + accessories
Valid Article
Skin graft mesher, Zimmer
Definition
A manual surgical device used to perforate a skin graft (a section of donor skin from another part of the patient's body) with a pattern of lacerations (cuts) before the skin is grafted to the area being treated on the patient. The skin may be placed on a skin graft carrier to better support it and will typically be run through an instrument with a row of cutting wheels, or be subjected to a rolling drum with a handle and cutting blades, to improve graft stretch and permeability and facilitate graft conformity and healing.
Synonym
skin graft expander
Specifications
Technical specifications
- STAGE
- made of stainless steel
- with handle and hinge system to attach the expander rollers
- guidance plateau with grooves for setting the dermacarriers
- dimensions: 16.5 cm x 18.4 cm
- weight: 3.2 kg
- RATCHET HANDLE
- dimensions: 22.2 x 3.2 cm
- EXPANDER ROLLER
- ratio 1 to 3 allowing to obtain a graft which is 3 times bigger than the initial cut off surface
- integrated comb mechanism which prevents the skin from sticking on the expander roller
- DERMACARRIER
- PVC tray, sterile, s.u., 10 cm wide
- 1 flat face on which the graft is placed
- 1 face with 2 grooves to be placed on the guidance platform
- comes in 2 lengths: 20.3 or 40.6 cm
- STERILIZATION CASE
Packaging & Labelling
- Skin graft mesher + roller + sterilization case: unit packaging
- Dermacarriers: individual sterile packaging + secondary packaging per pack of 10
Supplied with the Article
Skin graft mesher + roller ratio 1 to 3 + sterilization case.
To be Ordered Separately
The dermacarriers (two different sizes) have to be ordered separately (see related articles).
Instructions for use
See instructions for use.
Dermacarriers are for single use and must be discarded after use.
Maintenance
The skin graft mesher must be sterilized by steam autoclave after each use. Assemble and dismantle the components with great care and use a soft brush for cleaning before sterilization.
MSF requirements
Manual apparatus used to increase the surface of a skin graft (mesh graft) in order to cover a bigger surface of the destination site.
Selection of the expander roller with ratio 1 to 3 since it is the most used.
Single use sterile dermacarriers allowing collecting easily the graft by following the rules of asepsis.




![[KMEDKHOE1CO] OPERATING SUITE, PART med.equip. 2 operating rooms, complete](/web/image/product.template/572536/image_256/%5BKMEDKHOE1CO%5D%20OPERATING%20SUITE%2C%20PART%20med.equip.%202%20operating%20rooms%2C%20complete?unique=d05f4ef)
![[ESURDERW3--] DERMATOME, WATSON, manual, handle 30 cm](/web/image/product.template/573250/image_256/%5BESURDERW3--%5D%20DERMATOME%2C%20WATSON%2C%20manual%2C%20handle%2030%20cm?unique=1b305f9)
![[ESURMESGCA2] (skin graft mesher, Zimmer) CARRIER, sterile, s.u., 20.3 cm](/web/image/product.template/569155/image_256/%5BESURMESGCA2%5D%20%28skin%20graft%20mesher%2C%20Zimmer%29%20CARRIER%2C%20sterile%2C%20s.u.%2C%2020.3%20cm?unique=1736b9d)
![[ESURMESGCA4] (skin graft mesher, Zimmer) CARRIER, sterile, s.u., 40.6 cm](/web/image/product.template/569148/image_256/%5BESURMESGCA4%5D%20%28skin%20graft%20mesher%2C%20Zimmer%29%20CARRIER%2C%20sterile%2C%20s.u.%2C%2040.6%20cm?unique=1736b9d)
![[ESURMESGR13] (skin graft mesher, Zimmer) ROLLER ratio 1 to 3](/web/image/product.template/569150/image_256/%5BESURMESGR13%5D%20%28skin%20graft%20mesher%2C%20Zimmer%29%20ROLLER%20ratio%201%20to%203?unique=7af7e94)