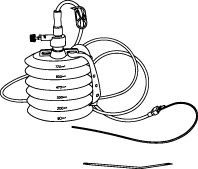
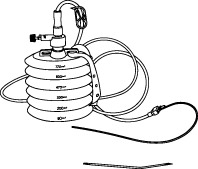
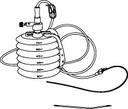
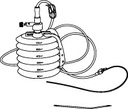
REDON, 400-600ml, + alene needle/drain CH 16, ster. s.u.
STD
SCTDREDP416
Valid Article
Account code:
60210
HS Code:
901890
Last Updated on:
12/04/2024, 17:35:41
Single use
A single-use device, also referred to as a disposable device, is intended for use on one patient during a single procedure. It is not intended to be reprocessed (i.e., cleaned and disinfected or sterilized).
A060101 - Vacuum and gravity drainage systems
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes
Thermosensitive codes are defined for storage and transportation temperature requirements of the products.
REDON, bellows
Definition
A collection of sterile devices designed to remove fluids or purulent material from a closed-wound in a controlled manner. It includes a container (the receptacle or reservoir), drains/catheters, connectors and a trocar for placing the drains/catheters into the site.
The reservoir is provided with markers at increments along its side to facilitate the approximate measurement of the collected fluids. It can be used for a wide range of surgical applications.
Specifications
Quality standards
ISO 20697, 2018, edition 1, (confirmed 2023) Sterile drainage catheters and accessory devices for single use
Technical specifications
Complete system including 4 items:
- REDON or ALENE NEEDLE
- austenitic stainless steel containing molybdenum
- solid curved needle: straight part +/-15 cm, curved part +/- 19 cm
- distal end with taper or cutting point
- PVC point protection
- threaded base enabling it to be screwed to the drain or attached to the drain
- REDON DRAIN
- soft PVC, kink resistant
- with radio-opaque line
- multiperforated distal (patient) end over 7 - 15 cm
- proximal end connected to the connecting tube
- overall length: ± 50 cm
- external diameter: CH12 or CH16
- CONNECTING TUBE
- PVC, etc.
- connects the drain to the collecting bottle
- fitted with a clamp
- length: ± 100 cm
- COLLECTING BOTTLE
- polyethylene, etc.
- accordion-shaped
- graduated
- capacity: 450 - 600 ml
- fitted with a non-return device
The whole system is sterile and for single use.
Packaging & Labelling
Unit sterile packaging in peel-open pack
Instructions for use
Mechanical device used for the active drainage of surgical wounds through negative pressure.
Storage
- Store below 30°C
- Protect from sunlight ‐ Protect from humidity
Some restricted information has been hidden. Sign in
to see this information