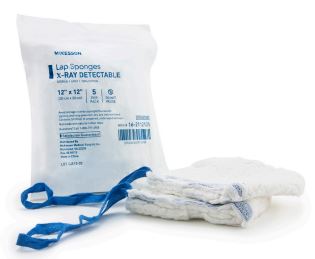
COMPRESS, abdominal, 45x45 cm, 4 layers, 17 threads, sterile
STD
SDRECOMP4A4
Valid Article
Account code:
60210
HS Code:
300590
Last Updated on:
12/04/2024, 16:30:27
Single use
A single-use device, also referred to as a disposable device, is intended for use on one patient during a single procedure. It is not intended to be reprocessed (i.e., cleaned and disinfected or sterilized).
M0201030201 - Laparotomy cotton gauzes, with x-ray detectable thread, sterile
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes
The product is part of at least one Kit.
A kit is a collection of products (medical and/or logistic) that are needed for a certain intervention in emergency. The choice and quantity of the articles reflects the MSF protocols for this specific situation. The use of Kits allows to start an intervention without a detailed evaluation.
COMPRESS, abdominal
Definition
Sterile thick and large compress, fitted with a loop and an X-ray contrast strip sewn in and stabilizing seams. Laparotomy swabs are intended for direct use in the operating room. They are used for holding and retaining organs and tissues as well as for haemostasis and absorbing body fluids during surgery. They are intended for short term use (up to 24 hours)
Synonym
laparotomy sponge
Specifications
Quality standards
EN 14079, 2003, Non-active medical devices - Performance requirements and test methods for absorbent cotton gauze and absorbent cotton and viscose gauze
Technical specifications
- Absorbent gauze, woven, 100 % pre-washed cotton
- Does not contain any active substance
- 17 - 20 threads/cm²
- Weight: 23 - 27 g/m²
- Size: unfolded = 45 cm x 45 cm +/- 1 cm, folded = 11.5 x 11.5 cm +/- 5mm
- 4 or 6 stitched layers
- X-ray detectable strip (PVC + barium sulfate)
- Turned in, overcast edges, ensuring high strength and no free thread
- Coloured loop for holding
- Sterile, for single use
Packaging & Labelling
Presentation in double packaging either 2 peelable pockets or a Pasteur folded crepe paper and a peelable pouch. Some packages may contain a label for tracability.
Some restricted information has been hidden. Sign in
to see this information
Some restricted information has been hidden. Sign in
to see this information







![[KMEDMHIS22-] (mod ICU) SPECIFIC DRESSINGS complementary 2021](/web/image/product.template/574343/image_256/%5BKMEDMHIS22-%5D%20%28mod%20ICU%29%20SPECIFIC%20DRESSINGS%20complementary%202021?unique=873d241)
![[KMEDKHAS2CO] AMP, PART medicines & renewable supplies, compulsory](/web/image/product.template/572649/image_256/%5BKMEDKHAS2CO%5D%20AMP%2C%20PART%20medicines%20%26%20renewable%20supplies%2C%20compulsory?unique=526b36a)
![[KMEDKHES2CO] EMERGENCY ROOM, PART medicines & ren.supplies, compulsory](/web/image/product.template/572657/image_256/%5BKMEDKHES2CO%5D%20EMERGENCY%20ROOM%2C%20PART%20medicines%20%26%20ren.supplies%2C%20compulsory?unique=526b36a)
![[KMEDKHAS2--] AMP, PART medicines & renewable supplies](/web/image/product.template/573083/image_256/%5BKMEDKHAS2--%5D%20AMP%2C%20PART%20medicines%20%26%20renewable%20supplies?unique=526b36a)
![[KMEDKHES2--] EMERGENCY ROOM, PART medicines & ren.supplies, with options](/web/image/product.template/573055/image_256/%5BKMEDKHES2--%5D%20EMERGENCY%20ROOM%2C%20PART%20medicines%20%26%20ren.supplies%2C%20with%20options?unique=fdd3a1b)
![[KMEDMHOS22-] (mod OT Room) DRESSINGS 2015](/web/image/product.template/572852/image_256/%5BKMEDMHOS22-%5D%20%28mod%20OT%20Room%29%20DRESSINGS%202015?unique=49d2a38)