FEMALE CONDOM, lubricated, 170 mm, s.u.
STD
SMSUCONDF1-
Valid Article
Account code:
60210
HS Code:
401410
Last Updated on:
12/04/2024, 18:18:34
Single use
A single-use device, also referred to as a disposable device, is intended for use on one patient during a single procedure. It is not intended to be reprocessed (i.e., cleaned and disinfected or sterilized).
22.1.4 - Barrier methods
Classification of the medicines in groups and subgroups according to their therapeutic use.
The classification used by MSF is based on the WHO Model List of Essential Medicines.
U110203 - Female condoms
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes
Thermosensitive codes are defined for storage and transportation temperature requirements of the products.
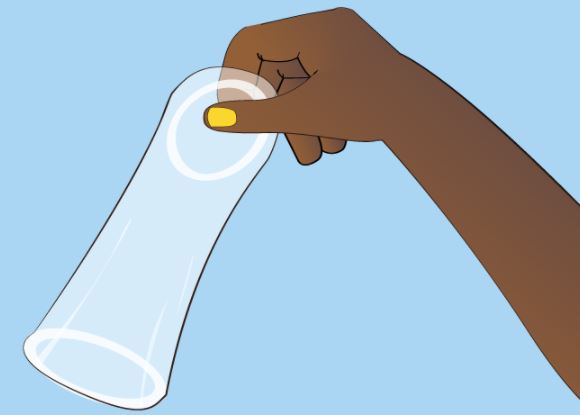
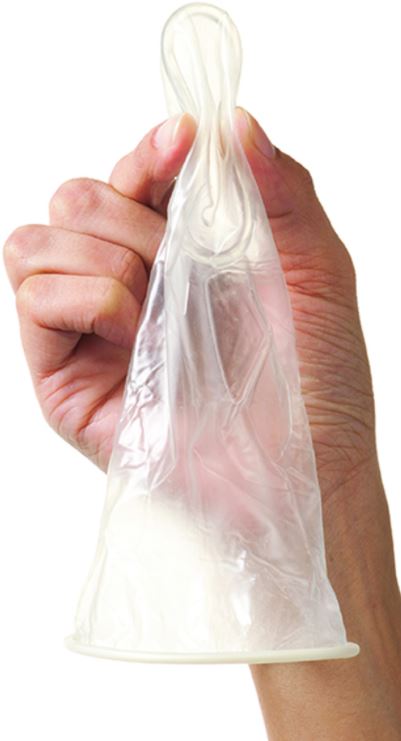
FEMALE CONDOM
Definition
Cylindrical, soft, thin, transparent sheath, lubricated, with flexible ring at each end.
The smaller inner ring, at the closed end, helps with insertion and secures the condom during intercourse.
The larger outer ring remains outside the vagina to anchor the condom so that the sheath covers the vulva as well as the base of the penis during intercourse.
Used for protection against sexually transmitted infections and for contraception.
Specifications
Some restricted information has been hidden. Sign in
to see this information
Quality standards
- WHO/UNFPA, 2012, female condom generic specification. Prequalification and guidelines for procurement
- ISO 25841, 2017, edition 3, Female condoms - Requirements and test methods
Technical specifications
Meets the WHO specifications:
- Sheath and outer ring made of nitrile
- Inner ring made of polyurethane
- Silicone-based lubricant
- Shape: cylindrical
- Smooth texture
- Colourless, odourless, transparent
- Non-irritating
- Length: 163-183 mm
- Width: 76-83 mm at its widest diameter
- Thickness: 65-85 µ
- Tensile strength: minimum 10.3 MPa
- Burst strength: minimum 3.2 litres
- Water leakage: no visible hole
- Non sterile, for single use
Packaging & Labelling
Individually packed in a sealed plastic envelope
Instructions for use
Some restricted information has been hidden. Sign in
to see this information
Precautions for Use
Never use a condom if the individual package is damaged or defective.
Storage
Store between 2° and 40º C, Protect from sunlight ‐ Protect from humidity
Some restricted information has been hidden. Sign in
to see this information