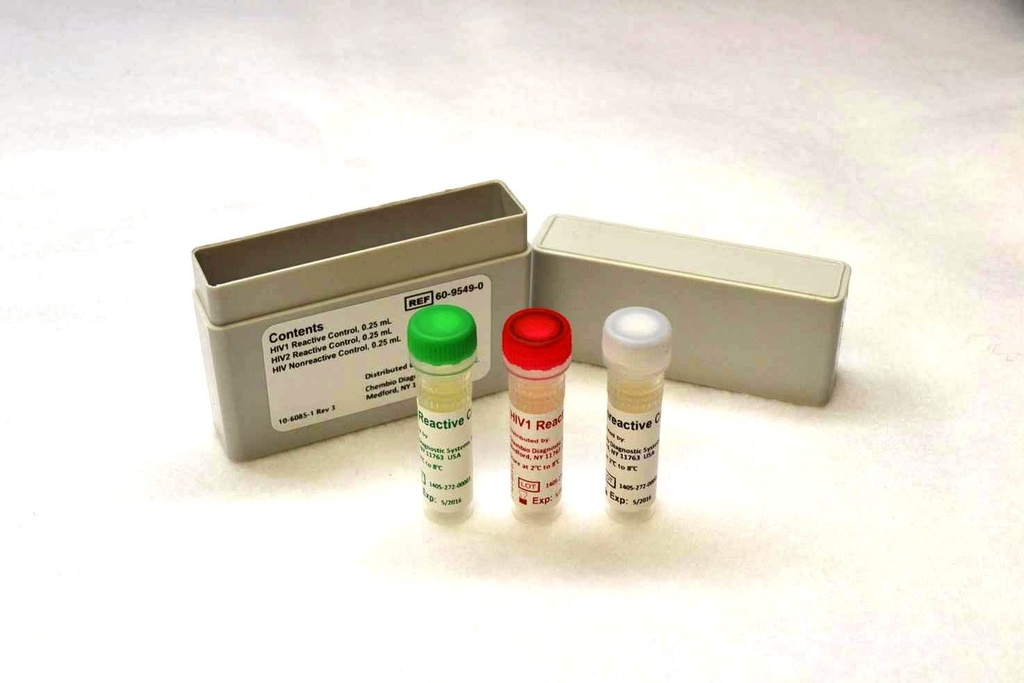
(test HIV 1+2 Stat-Pak) CONTROLS 3 x 0.25 ml, 60-9549-0
STD
SSDTHIVS201
Valid Article
Account code:
60200
HS Code:
382219
Last Updated on:
22/11/2024, 22:46:22
Former
Code(s):
DDGTHIVS201
Single use
A single-use device, also referred to as a disposable device, is intended for use on one patient during a single procedure. It is not intended to be reprocessed (i.e., cleaned and disinfected or sterilized).
W0105094001 - Controls infectious immunology/nat - rt & poc
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
Thermosensitive codes are defined for storage and transportation temperature requirements of the products.
The product is part of at least one Kit.
A kit is a collection of products (medical and/or logistic) that are needed for a certain intervention in emergency. The choice and quantity of the articles reflects the MSF protocols for this specific situation. The use of Kits allows to start an intervention without a detailed evaluation.

![[KMEDMTRA03B] MODULE, TRANSFUSION, children, part 2, cold chain](/web/image/product.template/569933/image_256/%5BKMEDMTRA03B%5D%20MODULE%2C%20TRANSFUSION%2C%20children%2C%20part%202%2C%20cold%20chain?unique=904b96e)
![[KMEDMTRA01B] MODULE, TRANSFUSION, 50, part 2, cold chain](/web/image/product.template/569077/image_256/%5BKMEDMTRA01B%5D%20MODULE%2C%20TRANSFUSION%2C%2050%2C%20part%202%2C%20cold%20chain?unique=8746124)
![[SSDTHIVS20T] HIV 1 + 2 TEST (STAT-PAK), ser/pl/wb, 1 test, HIV101](/web/image/product.template/569623/image_256/%5BSSDTHIVS20T%5D%20HIV%201%20%2B%202%20TEST%20%28STAT-PAK%29%2C%20ser-pl-wb%2C%201%20test%2C%20HIV101?unique=3f25a3f)