PREGNANCY RST/hCG TEST, urine, 1 strip
STD
SSDTPREG1S-
Valid Article
Account code:
60200
HS Code:
382290
Last Updated on:
29/11/2025, 22:10:40
Former
Code(s):
DDGTPREG1S-
Single use
A single-use device, also referred to as a disposable device, is intended for use on one patient during a single procedure. It is not intended to be reprocessed (i.e., cleaned and disinfected or sterilized).
CE marking: declaration that the product meets EU standards for health, safety, and environmental protection. The CE marking indicates that the product may be sold freely in any part of the European Economic Area, regardless of its country of origin.
20
Shelf life = length of time a product can remain active and effective.
Short shelf life = shelf life = < 24 months
W0102160302 - Hcg - rt & poc
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
Thermosensitive codes are defined for storage and transportation temperature requirements of the products.
The product is part of at least one Kit.
A kit is a collection of products (medical and/or logistic) that are needed for a certain intervention in emergency. The choice and quantity of the articles reflects the MSF protocols for this specific situation. The use of Kits allows to start an intervention without a detailed evaluation.
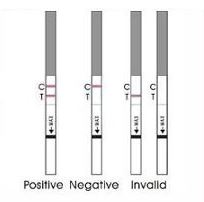
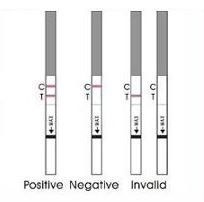
PREGNANCY RST/hCG TEST
Definition
Immunochromatographic test for the qualitative detection of hCG pregnancy hormone (human Chorionic Gonadotrophin) in urine.
Specifications
Technical specifications
- One-step rapid test (< 15 minutes) without any additional reagent
- Sample type: urine
- Sample volume: sufficient to immerge the strip
- Minimal sensitivity: 25 mUI/ml
Packaging & Labelling
Individually packed strips
Instructions for use
Always refer to the instructions for use, but in general:
- Preferably use the first morning urine as it contains higher hCG concentration.
- Read the results respecting the maximal time as expressed in the instructions
- The test can be done on the first day of a missed menstrual period. The hCG level reaches a peak 8 - 12 weeks after the last menstrual period and then declines to lower values.
- If pregnancy is still suspected after a negative result, a second test should be done after 48 - 72 hours.
Please consult the “Updated laboratory procedures, 2022” available online via the Laboratory working Group sharepoint page: Laboratory Procedures and Resources.
https://msfintl.sharepoint.com/sites/msfintlcommunities/LabWG/SitePages/Laboratory-Manual-page.aspx
For offline access, contact your laboratory advisor.
Storage
- Keep between 4 and 30°C in sealed pouch
- Exposure to temperatures over 30°C can damage the product
- Do not freeze, Protect from sunlight ‐ Protect from humidity
- Shelf life: 20 months
- Guaranteed minimum remaining shelf life at delivery: 1/3 of total shelf life
MSF requirements
No need for cold chain.
Some restricted information has been hidden. Sign in
to see this information




![[KMEDMHLA11-] (mod hospital lab) RAPID DIAGN. TESTS + GLUCOMETER +HEMOCUE](/web/image/product.template/572569/image_256/%5BKMEDMHLA11-%5D%20%28mod%20hospital%20lab%29%20RAPID%20DIAGN.%20TESTS%20%2B%20GLUCOMETER%20%2BHEMOCUE?unique=49b9e10)
![[KMEDMHMI23A] (mod hospital divers) MEDICINES RAPE MNGT., 35ad/15c part A](/web/image/product.template/574388/image_256/%5BKMEDMHMI23A%5D%20%28mod%20hospital%20divers%29%20MEDICINES%20RAPE%20MNGT.%2C%2035ad-15c%20part%20A?unique=0f37d38)
![[KMEDMHLA111] (mod hospital lab) RAPID DIAGNOSTIC TESTS](/web/image/product.template/572567/image_256/%5BKMEDMHLA111%5D%20%28mod%20hospital%20lab%29%20RAPID%20DIAGNOSTIC%20TESTS?unique=03d167f)
![[KMEDMSUP05P] (IEHK 2024 suppl. module) SUPPL. PEP UNIT, 50 ad. + 10 ch.](/web/image/product.template/583181/image_256/%5BKMEDMSUP05P%5D%20%28IEHK%202024%20suppl.%20module%29%20SUPPL.%20PEP%20UNIT%2C%2050%20ad.%20%2B%2010%20ch.?unique=7358754)
![[STSSCONT6U-] CONTAINER, SAMPLE, plast., 60ml, non sterile, urine](/web/image/product.template/570465/image_256/%5BSTSSCONT6U-%5D%20CONTAINER%2C%20SAMPLE%2C%20plast.%2C%2060ml%2C%20non%20sterile%2C%20urine?unique=8ec7222)