TEST, HEPATITIS E (Assure HEV), ser/pl/wb, 1 test 0743160020
Valid Article
HEPATITIS E TEST (Assure HEV)
CAUTION
This test is worded and codified by unit to make the order easier, but it is always packaged in kit of 20.
Definition
Specifications
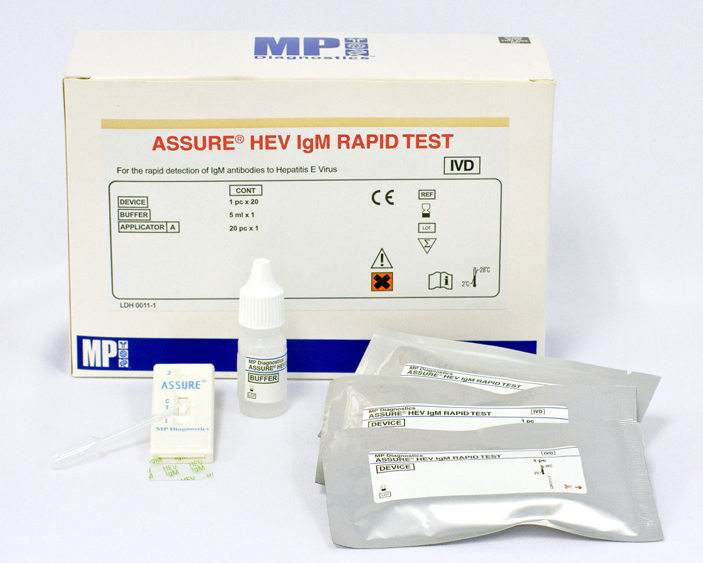
Components
- 20 x HEV IgM Rapid Test devices in individually sealed pouches with desiccant.
- 1 x (5 ml) chase buffer: containing 0.02% thimerosal and 0.01% Triton-X 100.
- 20 x plastic sample applicators: each with marks at 25µl and 35µl
- Instructions For Use
Technical specifications
- Sample: 25μl serum, plasma and 35μl whole blood
- Time: 15 min
Packaging & Labelling
Box of 20 individually sealed pouches each containing a test with desiccant.
To be Ordered Separately
- Safety blood lancet
- Alcohol swabs
Instructions for use
Use on serum, plasma or whole blood.
Follow the instructions on in the leaflet.
Precautions for Use
All biological samples must be considered as potentially infectious and handled with the usual precautions (compulsary wearing of gloves, hand washing, etc.)
Storage
- Store between 2-28°C
- Test devices should be kept sealed until use
- Not to be used beyond expiry date
- Shelf life: 23 months
- Guaranteed minimum remaining shelf life at delivery: 1/3 of total shelf life
Waste management
Presence of mercury in the reagents as thimerosal 0.02%. Please contact your watsan referent for advice on proper disposal.
Detailed hazard and precautionary information can be found in the safety data sheet (SDS).
Chase buffer containing 0.02% thimerosal and 0.01% Triton-X 100.
Signal Word
Warning
| H319 | Causes serious eye irritation |
| P264 | Wash … thoroughly after handling |
| P280 | Wear protective gloves/protective clothing/eye protection/face protection |
MSF requirements
Due to performance limitations, the test is intended for investigation of epidemics only, and not for individual diagnosisof Hepatitis E.