UNIVERSAL TRANSPORT MEDIUM+SWAB, 3ml, synth. mini tip
Valid Article
UNIVERSAL & VIRAL TRANSPORT MEDIUM + SWAB
Definition
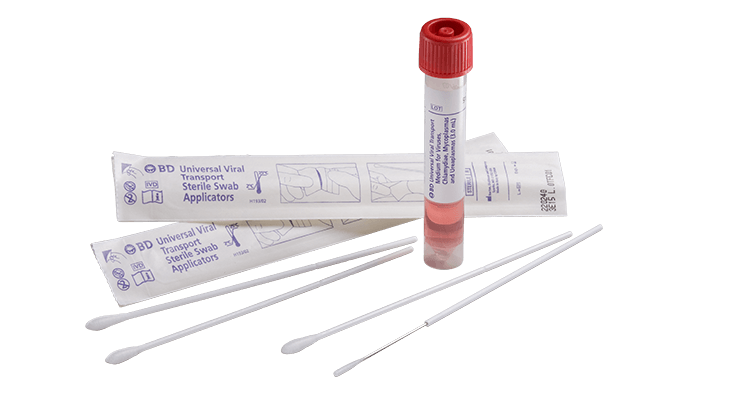
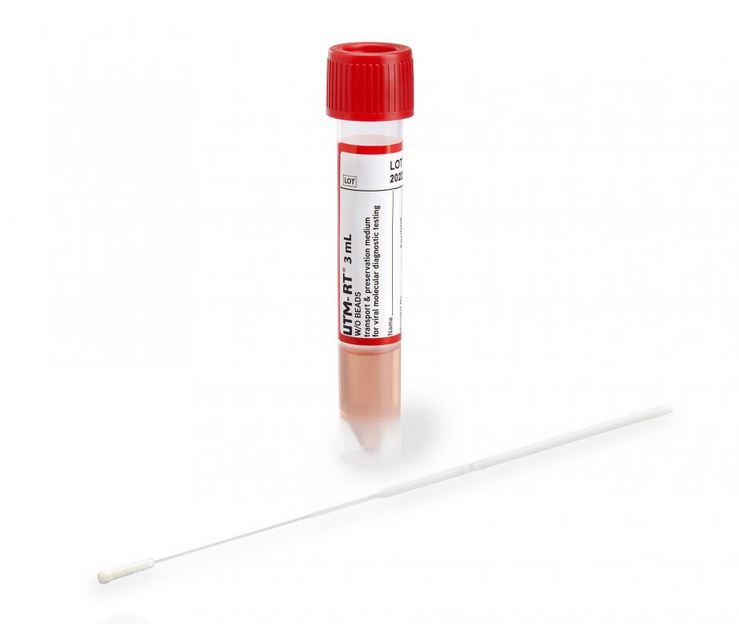
Kit containing Universal / Viral Transport Medium and 1 swab for the collection and transport of specimens containing viruses, chlamydiae, mycoplasmas or ureaplasmas from the collection site to the testing laboratory.
Specifications
- 3 ml of UTM medium in screw-cap tube
- One swab with synthetic tip and plastic handle with breaking point
Different presentations depending your ESC:
- Option 1: Supplied togehter, in kit or in bulk (STSSTRTTUUTM1)
- Option 2: Supplied toghether in bulk UTM (STSSTRTUUTM6) and swabs (STSSSWAB8PS)
Caution
In the exceptional situation that the references above are not available, alternative sources may be proposed. All specifications below should be met:
- Swab
- Swab with polysester/Rayon/Nylon tip, ideally flocked (not cotton!)
- Mini-tip, suitable for nasopharyngeal sample collection (for COVID)
- 1 swab/patient
- Plastic flexible stick/handle
- Sterile
- Viral transport medium
- 1,2 or 3 ml Viral transport medium isotonic to mammalian host cells, supplemented with antibiotics to inhibit growth of competing bacteria and yeast.
- Note that for analysis using the GeneXpert SARS-CoV-2 cartridge only the 3ml volume has been validated.
- Should not contain Guanidine Thiocyanate (= toxic)
Both to comply with CLSI standard M40-A
Technical specifications
- Medium Composition
- Hanks' Balanced Salts
- bovine serum albumin
- L-cysteine, gelatin
- sucrose
- L-glutamic acid
- HEPES buffer
- vancomycin
- amphotericin B
- colistin
- phenol red
- Mini-tip swabs are suitable for nasopharyngeal sample collection (whereas swabs with standard tips are for oropharyngeal sample collection)
Instructions for use
Please consult the “Updated laboratory procedures, 2022” available online via the Laboratory working Group sharepoint page: Laboratory Procedures and Resources.
https://msfintl.sharepoint.com/sites/msfintlcommunities/LabWG/SitePages/Laboratory-Manual-page.aspx
For offline access, contact your laboratory advisor.
Storage
- Temperature between 2 and 25°C.
- Do not overheat or freeze prior to use
- The product must be stored in its original packaging
- Shelf life: 16 months
- Guaranteed minimum remaining shelf life at delivery: 1/3 of total shelf life
MSF requirements
Sample collection and storage of the sample during transport to the laboratory, for suspiscions of viruses, chlamydiae, mycoplasmas or ureaplasmas.