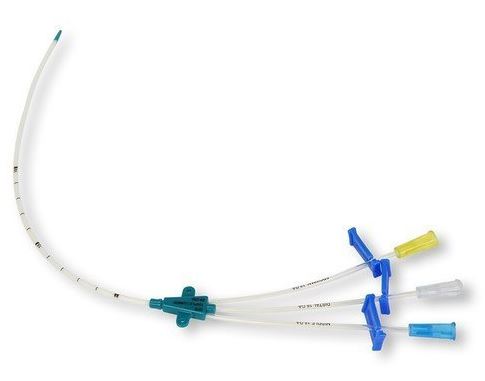
CENTRAL VENOUS CATHETER SET, 2 lumens, CH4-4.5 x 8-10cm
Valid Article
CENTRAL VENOUS CATHETER SET
Definition
A sterile, flexible tube intended to be introduced into the subclavian or the jugular vein and advanced into the superior vena cava for various infusion procedures including the intravenous administration of fluids or drugs, and blood transfusion. The proximal end of this central venous catheter (CVC) is fixed to the patient for long-term use. It includes supportive devices associated with introduction.
Specifications
Quality standards
- EN ISO 10555-1, 2013, edition 3, +A1 2017 Sterile, single-use intravascular catheters - Part 1: General requirements
- ISO 10555-3, 2013, edition 2, (confirmed 2018) Intravascular catheters - Sterile and single-use catheters - Part 3: Central venous catheters
Technical specifications
Non-tunneled catheters are fixed in place at the site of insertion, with the catheter and attachments protruding directly. (Tunneled catheters are passed under the skin from the insertion site to a separate exit site.)
The set is sterile, for single use and consists of:
- central venous catheter with extension tubes, a clamp and needle-free connector per lumen
- double lumen (SINSCVCAD+++) or triple lumen (SINSCVCAT+++)
- soft catheter tip
- X-ray detectable, opaque polyurethane
- length markings for accurate catheter placement
- no antibacterial components included in the catheter material
- Material for insertion (Seldinger technique): varies according the manufacturer
- kink-proof guide wire (stainless steel or nitinol) with advancer and flexible J-tip
- percutaneous insertion needle
- subcutaneous needle
- syringes: Luer-lock 5ml, Luer-slip 3 and 10 ml
- medicine cup / galipot x 2
- needle holder
- scalpel (safety version is preferred)
- antiseptic sponge swab
- gauze
- fixation dressing
- drape adhesive with transparent fenestration
| code | external diameter | length | use for |
SINSCVCAT720 | CH 7 - 7.5 | 15 - 20 cm | adults |
| SINSCVCAD511 | CH 5 - 5.5 | 10 - 13 cm | children |
| SINSCVCAD408 | CH 4 - 4.5 | 8 - 10 cm | newborns |
Packaging & Labelling
Sterile set in a tray peel open pack.
Instructions for use
- The location of the vein is identified with the use of an ultrasound device. A hollow needle is advanced through the skin until blood is aspirated.
- The line is then inserted using the Seldinger technique: a blunt guidewire is passed through the needle, which is then removed. A dilating device may be passed over the guidewire to expand the tract. Finally, the central line itself is then passed over the guidewire, which is then removed.
- All the lumens of the line are aspirated (to ensure that all are positioned inside the vein) and flushed with either saline or heparin.
- A chest X-ray may be performed afterwards to confirm that the line is positioned inside the superior vena cava and no pneumothorax was caused inadvertently.
Precautions for Use
Guidelines for the prevention of Intravascular Catheter-Related Infection (CDC 2002)
- Hand hygiene:
- before and after palpating catheter injection sites
- before and after inserting, replacing, accessing, repairing or dressing an intravascular catheter.
- Aseptic technique during catheter insertion and care: sterile gloves should be worn for the insertion of central catheters
- Sterile dressing for the central venous line. Changing of the dressing depends on the type of dressing:
- At least every 7 days for transparent dressings
- Every 2 days for gauze dressings
- All dressings should be changed when loose or soiled
- Replace administration sets no more frequently than at 72-hour interval.
- Clean injection ports with chlorhexidine 2% / alcohol 70% before use: scrub the port creating friction for 15-30 seconds. Use different parts of the wipe/gauze, allow to dry
MSF requirements
Use in ICU for long term venous access, monitoring of central venous pressure, infusion of certain medications.
All-in-one package is preferred.



![[KMEDMHIS24-] (mod ICU) INJECTION SUPPLIES 2021](/web/image/product.template/574424/image_256/%5BKMEDMHIS24-%5D%20%28mod%20ICU%29%20INJECTION%20SUPPLIES%202021?unique=cd1d7c2)
![[DEXTCHLHA2W] CHLORHEXIDINE 2%, 70% isopropyl alcohol, SWAB/WIPE](/web/image/product.template/570781/image_256/%5BDEXTCHLHA2W%5D%20CHLORHEXIDINE%202%25%2C%2070%25%20isopropyl%20alcohol%2C%20SWAB-WIPE?unique=1c75ce3)
![[EDIMULSC003] (ultrasound) COUPLING GEL, sterile, 20ml, sachet](/web/image/product.template/571681/image_256/%5BEDIMULSC003%5D%20%28ultrasound%29%20COUPLING%20GEL%2C%20sterile%2C%2020ml%2C%20sachet?unique=ec181af)
![[EDIMULSC412] (ultrasound) COVER + GEL transducer, 14x120cm, ster., s.u.](/web/image/product.template/574472/image_256/%5BEDIMULSC412%5D%20%28ultrasound%29%20COVER%20%2B%20GEL%20transducer%2C%2014x120cm%2C%20ster.%2C%20s.u.?unique=8d36328)
![[SDREFIDS1IVL] FILM DRESSING, semi-permeable, adhesive, IV, sterile, L](/web/image/product.template/568824/image_256/%5BSDREFIDS1IVL%5D%20FILM%20DRESSING%2C%20semi-permeable%2C%20adhesive%2C%20IV%2C%20sterile%2C%20L?unique=b7e0424)
![[SINSIVCH1--] INTRAVENOUS CATHETER HOLDER, sterile, s.u., universal](/web/image/product.template/569590/image_256/%5BSINSIVCH1--%5D%20INTRAVENOUS%20CATHETER%20HOLDER%2C%20sterile%2C%20s.u.%2C%20universal?unique=ebcb97f)
![[SINSNLVCSN1] NEEDLELESS CONNECTOR, split-septum, neutral, sterile, s.u.](/web/image/product.template/569591/image_256/%5BSINSNLVCSN1%5D%20NEEDLELESS%20CONNECTOR%2C%20split-septum%2C%20neutral%2C%20sterile%2C%20s.u.?unique=608c7cc)