(warming unit) WARMING BLANKET, infant ref. 92257
Valid Article
(warming unit) WARMING BLANKET, reusable, NST
Please always consider the MSF STD, EEMDWAUA001 WARMING BLANKET, adult 3/4 reusable, before ordering a NST article.
Definition
Reusable device intended to help prevent a patient from cooling prior to, during, and after a surgical intervention by serving as a heat-generating cover.
The blanket must be connected to a warm air generator
Specifications
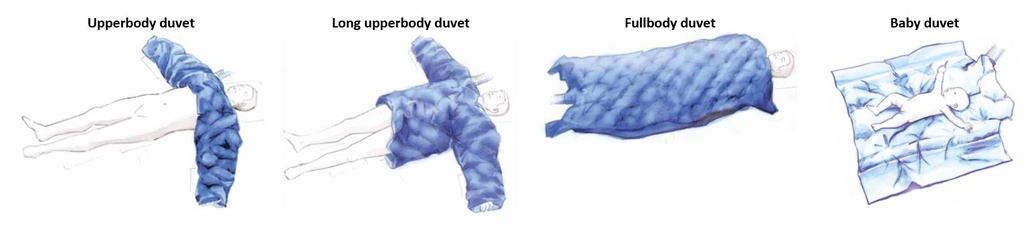
EEMDWAUA002: Upperbody duvet:
- Covers chest, arms and shoulders during surgical procedures on the lower part of the body.
- Size approx. 70 x 210 cm.
- Weight approx. 300 g.
EEMDWAUA003: Long upperbody duvet
- Covers chest, stomach, arms and shoulders during surgical procedures on the lower part of the body.
- Size approx. 120 x 210 cm.
- Weight approx. 400 g.
EEMDWAUA005 : Fullbody duvet
- Covers whole body for surgery in neck and head or for recovery and intensive care.
- Size approx. 110 x 200 cm.
- Weight approx. 500 g.
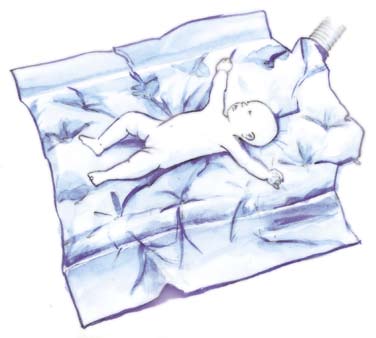
EEMDWAUA004 : Baby duvet
- Small duvet for surgery on babies and small children
- Size approx. 90 x 110 cm.
- Weight approx. 200 g.
Material
Polyester micro fibre: MicroPES and Polyurethane coated MicroPES. MicroPES fibres have Oekotex approval class 1.
Technical specifications
- Washable, non allergenic material
- Autoclavable
- Made from woven textiles that will not liberate fluffs or other particles:
- patient's side: polyester micro fibres (MicroPES)
- outer side: polyurethane coated polyester micro fibres
- Designed with integrated pockets for the warm air circulation
- Outlet to connect the hose of the warming unit with elastic strap
- Blanket must only be connected to warm air generator with maximum air flow 100 m3/ hour and maximum air temperature 46° C
- Reusable (can be reused at least 150 times)
- Non sterile
Instructions for use
The light blue side of the blanket shall always be used towards the patient.
The dark blue side is the outer side.
Precautions for Use
- Monitor the patient's body temperature at regular intervals.
- Do not use on ischaemic limbs, as heat injuries may occur.
- Never obstruct the patient's free respiration with the blanket.
Maintenance
- Wash before first use and after each use in a washing machine at max 95°C.
- Wash separately the first 2 washes.
- Tumble dryer at max. 120°C.
- Autoclave at max. 134°C / 2 bar.
- Iron at low temperature (synthetic material).
- Do not dry-clean and do not chlorine bleach.
MSF requirements
The blanket shall be used for the optimization of patient's body temperature during surgical interventions or in the emergency room. It is reusable, easy to clean, soft and comfortable, easy to form around the patient.
Reduces risk factors during anaesthesia such as arrhythmia, prolonged awakening, hypo- or hyperthermia and cold shivering.


![[EEMDWAUA001] (warming unit) WARMING BLANKET, adult 3/4 reusable 92057](/web/image/product.template/571725/image_256/%5BEEMDWAUA001%5D%20%28warming%20unit%29%20WARMING%20BLANKET%2C%20adult%203-4%20reusable%2092057?unique=020d0fb)