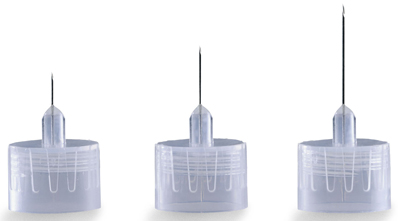
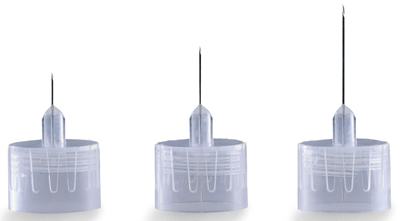
PEN NEEDLE, 31-32G, 0.23-0.25 x 4-5mm
Valid Article
AUTO INJECTOR NEEDLE
Definition
A device designed for the parenteral administration of a drug contained in a cartridge that forms part of, is attached to, or inserted into an autoinjector (drug pen injector).
It is used by persons requiring regular, self-administrated dosages of insulin, hormones, or other pharmaceuticals.
This device will typically be constructed as a double-ended, stainless steel needle of various sizes that is fixed to a threaded hub of plastic to which the drug pen injector is connected.
This device is packaged in a sealed sterility barrier. This is a single-use device.
Synonym
pen needle
Specifications
Quality standards
- ISO 11608-2, 2022, edition 3, Needle-based injection systems for medical use — Requirements and test methods — Part 2: Double-ended pen needles
- ISO 7864, 2016, edition 4, (confirmed 2021) Sterile hypodermic needles for single use - Requirements and test methods
Technical specifications
- pen needle that fits all major injector pens
- Gauge: 28G - 32 G
- length: 4 mm - 12 mm
- with protection cap
- sterile, single use
Instructions for use
To be used with a disposable pen containing a prefilled amount of insulin. When this type of pen is empty, it is thrown away.
Must be given as deep SC injection only.
Preparation for administration:
- Wash hands.
- Check the pen label (type and strength of insulin, expiry date) and the solution (cloudy, white and aqueous).
- Stir the insulin by gently rolling the pen between the palms of the hands. Do not shake the pen.
- Remove the cap from the pen.
- Wipe the pen tip (rubber seal) with a disinfectant.
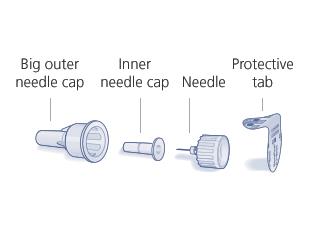
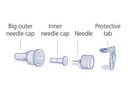
- Attach a pen needle to the pen after removing the protective seal of the needle. Push the needle straight onto the pen. Screw the needle on the pen.
- Remove the outer cap of the needle and save it (you will need it to remove the needle after the injection).
- Remove the inner cap of the needle.
- Remove air from the pen. Pointing the needle up in the air, select one or two units on the pen. Gently tap the pen to move air bubbles to the top of the pen.
- Press the injection button. A drop of insulin should appear on the tip of the needle. If no drop appears, change the needle and repeat this step.
- Select the correct dose on the pen. Turn the dose selector to the required number of units.
How to administer SC insulin:
- Insulin can be injected into the abdomen (two fingers away from the belly button), upper arm, buttocks, hips, buttocks, or the front or side of the thigh.
- Injecting insulin in the same area repeatedly can cause lumps, swelling and thickened skin, and it may keep insulin from absorbing properly. See the picture below on “rotating” injection sites.
- Do not inject insulin into areas that have wounds or bruising.
- Clean the chosen injection site with soap and water. Allow to dry.
- Pinch the skin.
- Keep the pen straight at a 90-degree angle to the skin and insert the needle with one quick motion.
- Push the injection button with the thumb to inject the dose of insulin. Wait for 10 seconds before releasing the button and removing the needle from the skin.
- Apply gentle pressure on the injection site with the finger for 5-10 seconds to keep insulin from leaking out.
- Remove the needle from the pen by replacing the outer needle cap on and unscrewing. Leaving the needle on the pen can result in leakage or air bubbles.
- Safety discard the needle into a sharps container.
- Replace the pen cap.
Precautions for Use
- check compatibility with the injector pen
- Make sure that along with rotating injection sites, you follow these rules for using needles correctly.
- Use new needles either for every injection or at least change them once a day.
- Do not inject through clothing
MSF requirements
for use with insulin pen







![[DINJINSAL3APS] INSULIN GLARGINE, LONG, 100 IU/ml, 3ml, pref. pen S](/web/image/product.template/583142/image_256/%5BDINJINSAL3APS%5D%20INSULIN%20GLARGINE%2C%20LONG%2C%20100%20IU-ml%2C%203ml%2C%20pref.%20pen%20S?unique=dc876c2)
![[DINJINSHB3APN] INSULIN HUMAN, BIPHASIC 30-70 UI/ml, 3ml, pref. pen N](/web/image/product.template/583140/image_256/%5BDINJINSHB3APN%5D%20INSULIN%20HUMAN%2C%20BIPHASIC%2030-70%20UI-ml%2C%203ml%2C%20pref.%20pen%20N?unique=a6d485a)
![[DINJINSHI3APN] INSULIN HUMAN, ISOPHANE (NPH) 100 UI/ml, 3ml, pref. pen N](/web/image/product.template/583143/image_256/%5BDINJINSHI3APN%5D%20INSULIN%20HUMAN%2C%20ISOPHANE%20%28NPH%29%20100%20UI-ml%2C%203ml%2C%20pref.%20pen%20N?unique=1d55fe3)
![[DINJINSHR3APN] INSULIN HUMAN, RAPID 100 IU/ml, 3ml, pref. pen N](/web/image/product.template/583144/image_256/%5BDINJINSHR3APN%5D%20INSULIN%20HUMAN%2C%20RAPID%20100%20IU-ml%2C%203ml%2C%20pref.%20pen%20N?unique=a6d485a)