(box, triple packag. UN3373) POUCH, PE, secondary packag. A4
STD
STSSUN62WB-
Valid Article
HS Code:
{}
Last Updated on:
12/04/2024, 16:04:36
Former
Code(s):
PPACZFR0207 PPACUN62WB- STSSWB-
Single use
A single-use device, also referred to as a disposable device, is intended for use on one patient during a single procedure. It is not intended to be reprocessed (i.e., cleaned and disinfected or sterilized).
W05020101 - Samples transport, pouches
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
(triple pack. UN3373) POUCH, PE, secondary packaging
Definition
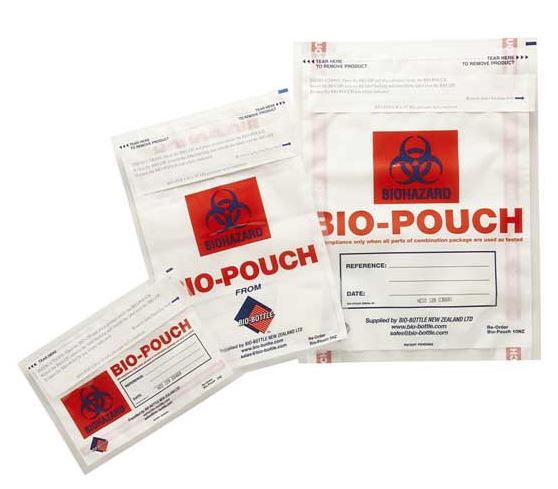
Disposable transparent pouch for infectious sample transport of class 6.2.
Secondary packaging intended for the transfer of infectious products of class 6.2, in particular diagnostic samples, code UN3373.
It bears the Biohazard sign and the words “BIOLOGICAL SUBSTANCE, CATEGORY B”.
Specifications
Technical specifications
- Transparent pouch
- Material: polyethylene
- Leak resistant
- Size: A4, 20 x 30 cm
- with Biohazard sign and the words “BIOLOGICAL SUBSTANCE, CATEGORY B”
- Adhesive closure
- Optional:
- Absorbent sheet of paper or cloth
- Document pocket
Instructions for use
This item should always be used with/ is included in:
- BOX, triple packaging, biological substance UN3373 + pouch (STSSUN62DS-)
- BOX ISOTHERMAL, triple packaging, biological substance UN3373 + pouch (STSSUN62DSI)
See related articles below.
Some restricted information has been hidden. Sign in
to see this information
MSF requirements
Transport of samples.
Suitable for use in shipments of diagnostic specimens classified as UN3373, as a secondary leakproof packaging. It must be used as part of a triple packaging and cannot be used alone.
Some restricted information has been hidden. Sign in
to see this information



![[STSSUN62DS-] BOX, triple packaging, biological substance UN3373 +pouch](/web/image/product.template/571091/image_256/%5BSTSSUN62DS-%5D%20BOX%2C%20triple%20packaging%2C%20biological%20substance%20UN3373%20%2Bpouch?unique=0a028ad)
![[STSSUN62DSI] BOX ISOTHERMAL, triple pack., biological subst.UN3373 +pouch](/web/image/product.template/571102/image_256/%5BSTSSUN62DSI%5D%20BOX%20ISOTHERMAL%2C%20triple%20pack.%2C%20biological%20subst.UN3373%20%2Bpouch?unique=0a028ad)