ADMINISTRATION SET EN, gravity, ENPlus/ENFit, ster.,s.u.
Valid Article
ADMINISTRATION SET, enteral nutrition, gravity
Definition
A device intended to enable connection of components of an enteral feeding system. It is typically a small plastic device containing a lumen to facilitate enteral administration of feeds after connection; it has no additional functionality and is dedicated to enteral feeding applications by gravity.
If a feeding pump is used, another specific administration set is needed (see enteral nutrition pump).
Specifications
Quality Standards Comment
See information onnasogastric and enteral tubes
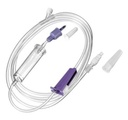
Technical specifications
- TUBING:
- Transparent PVC, DEHP free, latex free
- Ø 3.0 x 4.1 mm
- Length: 175 – 200 cm
- DROP-COUNTING CHAMBER
- THUMB WHEEL REGULATOR: Allows to adjust feeding flow rate between 0 and the maximal value.
- PROXIMAL CONNECTOR (to the feeding pouch):
- ENPlus
- non compatible with Luer
- ADMINISTRATION PORT: For medication, hydration and flushing of the tubing
- ENFit connection
- Located near the distal end
- DISTAL CONNECTOR (towards the gastric tube):
- ENFit
- non compatible with Luer
- COLOUR of the connections: purple is often used for enteral feeding medical devices, but the specific ISO norm does not specify the colour.
- STERILE
- FOR SINGLE USE: Bacterial contamination has been associated with the re-use of feed bags and administration sets. As evidence suggests re-use is not advisable, the administration system should be considered single use only and discarded after each intermittent feeding session.
Packaging & Labelling
Unit sterile packaging in peel-open pack
Instructions for use
Connect the set to the feed container as instructed on the packaging of the pump set.
Change the administration set for enteral feeding systems every 24 hours (open system) or 48 hours (closed system) or according to manufacturer's instructions.
IV giving sets have a drop-factor = number of drops of DISTILLED WATER / ml. The viscosity is an important factor in the determination of the number of drops /ml, and the enteral food is vary variable in viscosity. Hence it makes no sense to add a drop factor (calculated on water) to have a precise quantity of enteral food.
Precautions for Use
For patient safety, be aware that the feeding set tubing can get wrapped around a child's neck, which can lead to strangulation or death. (FDA safety communication Febr 2022)
Preventing misconnection errors
See information on nasogastric and enteral tubes
MSF requirements
Part of necessary materials for providing enteral nutrition without electricity (enteral feeding pump) to patients where oral intake of calories and/or proteins is not possible or not sufficient.




![[KMEDMNUTI33] (module nut. inpatient) MEDICAL SUPPLIES 2021](/web/image/product.template/575289/image_256/%5BKMEDMNUTI33%5D%20%28module%20nut.%20inpatient%29%20MEDICAL%20SUPPLIES%202021?unique=ce93f30)
![[KMEDMHIS25-] (mod ICU) CATHETERS, TUBES and DRAINS 2021](/web/image/product.template/574344/image_256/%5BKMEDMHIS25-%5D%20%28mod%20ICU%29%20CATHETERS%2C%20TUBES%20and%20DRAINS%202021?unique=aecada5)
![[KMEDMHCS211] (mod OPD) COMPLEMENTARY RENEWABLE SUPPLIES 2021](/web/image/product.template/574350/image_256/%5BKMEDMHCS211%5D%20%28mod%20OPD%29%20COMPLEMENTARY%20RENEWABLE%20SUPPLIES%202021?unique=4c1ecd0)
![[KMEDMHWS35-] (mod ward) CATHETERS, TUBES & DRAINS 2021](/web/image/product.template/574358/image_256/%5BKMEDMHWS35-%5D%20%28mod%20ward%29%20CATHETERS%2C%20TUBES%20%26%20DRAINS%202021?unique=e4f6df6)
![[NFOSENHAHPW05] ENTERAL RTU, hypercal. adult, high prot.,w/o fibre 0.5l](/web/image/product.template/572172/image_256/%5BNFOSENHAHPW05%5D%20ENTERAL%20RTU%2C%20hypercal.%20adult%2C%20high%20prot.%2Cw-o%20fibre%200.5l?unique=277d91d)
![[NFOSENIANPW05] ENTERAL RTU, isocal. adult, normoprot., w/o fibre 0.5l](/web/image/product.template/569345/image_256/%5BNFOSENIANPW05%5D%20ENTERAL%20RTU%2C%20isocal.%20adult%2C%20normoprot.%2C%20w-o%20fibre%200.5l?unique=b1517f8)
![[NFOSENICNPW05] ENTERAL RTU, isocal. child, normoprot, w/o fibre, 0.5l](/web/image/product.template/569985/image_256/%5BNFOSENICNPW05%5D%20ENTERAL%20RTU%2C%20isocal.%20child%2C%20normoprot%2C%20w-o%20fibre%2C%200.5l?unique=e4cc307)
![[SCTDTUGF06-] NASOGASTRIC TUBE, ENFit tip, s.u., 50 cm, CH06, light green](/web/image/product.template/571563/image_256/%5BSCTDTUGF06-%5D%20NASOGASTRIC%20TUBE%2C%20ENFit%20tip%2C%20s.u.%2C%2050%20cm%2C%20CH06%2C%20light%20green?unique=a9c2c0e)
![[SCTDTUGF08-] NASOGASTRIC TUBE, ENFit tip, s.u., 50 cm, CH08, blue](/web/image/product.template/571562/image_256/%5BSCTDTUGF08-%5D%20NASOGASTRIC%20TUBE%2C%20ENFit%20tip%2C%20s.u.%2C%2050%20cm%2C%20CH08%2C%20blue?unique=0fd4d08)
![[SCTDTUGF10-] NASOGASTRIC TUBE, ENFit tip, s.u., 50 cm, CH10, black](/web/image/product.template/571565/image_256/%5BSCTDTUGF10-%5D%20NASOGASTRIC%20TUBE%2C%20ENFit%20tip%2C%20s.u.%2C%2050%20cm%2C%20CH10%2C%20black?unique=a9c2c0e)
![[SCTDTUGF12-] NASOGASTRIC TUBE, ENFit tip, s.u., 90 cm, CH12, white](/web/image/product.template/570057/image_256/%5BSCTDTUGF12-%5D%20NASOGASTRIC%20TUBE%2C%20ENFit%20tip%2C%20s.u.%2C%2090%20cm%2C%20CH12%2C%20white?unique=e9f3722)
![[SCTDTUGF14-] NASOGASTRIC TUBE, ENFit tip, s.u., 90 cm, CH14, green](/web/image/product.template/570060/image_256/%5BSCTDTUGF14-%5D%20NASOGASTRIC%20TUBE%2C%20ENFit%20tip%2C%20s.u.%2C%2090%20cm%2C%20CH14%2C%20green?unique=254a5f3)