FILM DRESSING, semi-permeable, adhesive, IV, sterile, S
STD
SDREFIDS1IVS
Valid Article
Account code:
60210
HS Code:
300590
Last Updated on:
12/04/2024, 16:59:32
Single use
A single-use device, also referred to as a disposable device, is intended for use on one patient during a single procedure. It is not intended to be reprocessed (i.e., cleaned and disinfected or sterilized).
M04010202 - Polyurethane fixing dressings
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes
Thermosensitive codes are defined for storage and transportation temperature requirements of the products.
FILM DRESSING, semi-permeable, IV
Definition
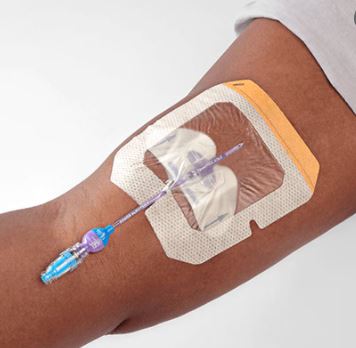
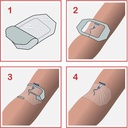
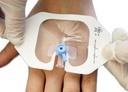
A sterile, transparent, adhesive and semi-permeable (i.e. impermeable to fluids, permeable to vapours and gases) covering. It is applied to secure to skin vascular catheters, or infusion ports and is intended to provide protection to fluids and external contaminants.
Specifications
Quality standards
EN 13726-2, 2002, Test methods for primary wound dressings. Moisture vapour transmission rate of permeable film dressings
Technical specifications
- Transparent film made of synthetic polymer (e.g., polyurethane based), latex- and PVC-free
- Breathable: allows moisture vapor and gas exchanges
- Moisture Vapour Transmission Rate ≥ 500g/m²/24h
- Waterproof: impervious to liquids and body fluids
- Protection against all external contamination
- Barrier against bacteria and viruses ≥20nm
- Adhesive:
- must adhere to the skin during 7 days minimum
- hypoallergenic latex-free adhesive
- With frame for easy positioning
- Documentation tape strip : preprinted for documenting dressing changes (optional)
- Securement tape strip to enhance stabilization of the catheter (optional but practical)
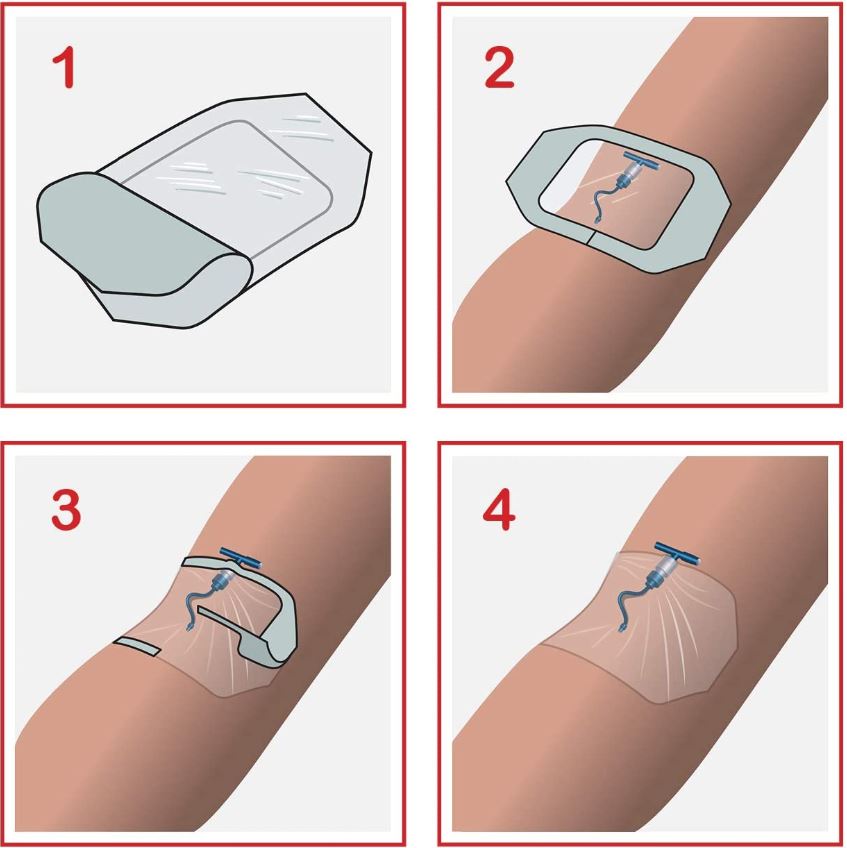
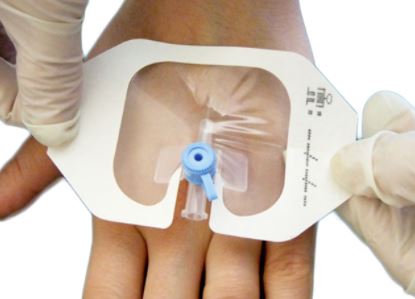
- Design of dressing allows aseptic application using a non-touch technique
- Split ensuring an efficient closure around the IV catheter
- Sterile, for single use
- Without anti-microbial agents
- Approximate size:
- small: 4 x 5 cm
- medium: 6 x 9 cm
- large: 10 x 11 cm
Packaging & Labelling
Each dressing is packed in a disposable peel pack allowing effective sterilization, safe handling, and storage until needed for use and facilitating proper aseptic presentation.
Instructions for use
Used to cover and protect catheter sites.
- Small: for pediatric use
- Large: use in critical contexts such as VHF or for Central venous catheters and PICC lines.
Precautions for Use
Storage
- Store below 25°C
- Protect from sunlight ‐ Protect from humidity
MSF requirements
Some restricted information has been hidden. Sign in
to see this information






![[KMEDMHIS22-] (mod ICU) SPECIFIC DRESSINGS complementary 2021](/web/image/product.template/574343/image_256/%5BKMEDMHIS22-%5D%20%28mod%20ICU%29%20SPECIFIC%20DRESSINGS%20complementary%202021?unique=873d241)
![[KMEDMNUTI33] (module nut. inpatient) MEDICAL SUPPLIES 2021](/web/image/product.template/575289/image_256/%5BKMEDMNUTI33%5D%20%28module%20nut.%20inpatient%29%20MEDICAL%20SUPPLIES%202021?unique=0b15096)
![[KMEDMNUTO33] (module nut. outpatient) MEDICAL SUPPLIES 2021](/web/image/product.template/575294/image_256/%5BKMEDMNUTO33%5D%20%28module%20nut.%20outpatient%29%20MEDICAL%20SUPPLIES%202021?unique=0b15096)