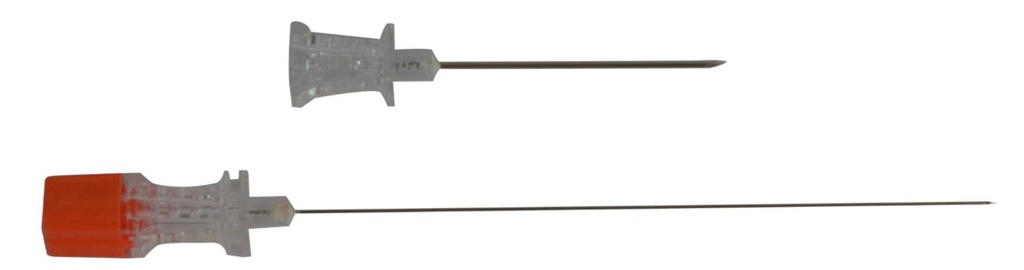
SPINAL NEEDLE, ANAESTHESIA, Luer, pencil, 25G x 90 mm, guide
Valid Article
SPINAL NEEDLE, ANAESTHESIA, pencil point
Definition
Hollow needle which is introduced into the subarachnoid space at the lumbar vertebral level to inject a local anaesthetic into the cerebrospinal fluid.
Synonym
Whitacre point spinal needle, pencil point spinal needle
Specifications
Quality standards
- ISO 7864, 2016, edition 4, (confirmed 2021) Sterile hypodermic needles for single use - Requirements and test methods
- ISO 6009, 2016, edition 4, (confirmed 2021) Hypodermic needles for single use - Colour coding for identification
Technical specifications
- NEEDLE
- Stainless steel, not coated with silicone
- Pencil tip (Whitacre): designed to separate the dural fibres instead of cutting them
- Translucent hub to observe CSF reflux with female Luer connector
- Notch on the hub, indicating the direction of the bevelled tip
- Protective sheath
- STYLET
- stainless steel, not coated with silicone
- closely fitted to the needle lumen
- hub with standardized colour code corresponding to the external diameter of the needle: orange for 25G
- INTRODUCTION GUIDE for the 25 G needle: the spinal needle is inserted through this introducer to reduce the risks of kinking
- stainless steel rod, not coated with silicone
- size: G 20: 0.9 mm x 32 - 38 mm
- hub
- Sterile, for single use
Comes in 2 lengths:
- SINSNESAP25I = 88 - 90 mm long
- SINSNESAP25IL = +/- 120 mm long and is intended for obese patients
Packaging & Labelling
Unit sterile packaging in peel-open pack.
Box of 10 or 25 pieces.
Instructions for use
Precautions for Use
To prevent accidental needlestick injuries, never recap the needle after use.
MSF requirements
The 22G rachi needles have been removed from the standard list as their use is associated with high prevalence of CSF leakage following dural puncture during spinal anaesthesia, resulting in high rates of post dural puncture headaches, particularly in obstetrics. The main size to be used is 25G and available. There is an over-use of the 22G needle in the field, although it can be useful in some cases of elderly patients with calcified intervertebral spaces, the overall risks outweigh this potential benefit.







![[KMEDMHOS33-] (mod OT Room) INJECTION SUPPLIES 2021](/web/image/product.template/574470/image_256/%5BKMEDMHOS33-%5D%20%28mod%20OT%20Room%29%20INJECTION%20SUPPLIES%202021?unique=bb39478)
![[SINSCONT2P-] SHARPS CONTAINER, 1 - 2 l, plastic, s.u.](/web/image/product.template/569684/image_256/%5BSINSCONT2P-%5D%20SHARPS%20CONTAINER%2C%201%20-%202%20l%2C%20plastic%2C%20s.u.?unique=affbf60)
![[SINSCONT4P-] SHARPS CONTAINER, 4 l, plastic, s.u.](/web/image/product.template/569672/image_256/%5BSINSCONT4P-%5D%20SHARPS%20CONTAINER%2C%204%20l%2C%20plastic%2C%20s.u.?unique=56d156f)