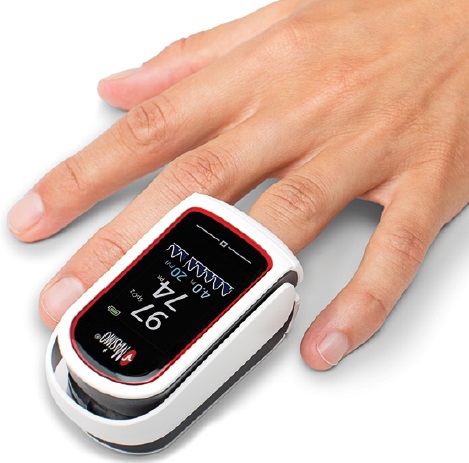
FINGERTIP PULSE OXIMETER (Mightysat 9707)
Valid Article
FINGERTIP PULSE OXIMETER (Mightysat 9707)
Definition
A portable, battery-powered, photoelectric device intended for the transcutaneous measurement and display of haemoglobin oxygen saturation (SpO2). The signals, typically produced by light-emitting diodes (LEDs) and a receiving detector in a probe, or directly built-in, are used to make the measurements using the principle of spectrophotometry.
The oximeter displays the SpO2 values and may calculate/display other parameters, e.g., pulse rate. The device is typically applied to the fingertip.
It may be used in Emergency rooms and outpatient departments to perform triage and collect vital signs (SpO2, HR; RR; perfusion index). In inpatient wards it allows to collect vital signs in a regular but non continuous manner.
Specifications
Quality standards
Technical specifications
- SpO2 and pulse monitor integrated into fingers/toe clip
- Configurations required to apply to
- adults and children
- all skin pigmentations
- Measurement description:
- Functional Oxygen Saturation (SpO2):
- detection range: 0 –100 %
- resolution: 1% or less
- accuracy in the range 70-99%: within +/-3%
- Pulse Rate (PR):
- detection range: 25–240 bpm
- resolution: 1bpm or less
- accuracy: +/- 3 bpm
- Perfusion Index (Pi): 0 .02–20%
- Display shows % SpO2, pulse rate, signal quality, sensor error or disconnect and low battery status.
- Audible and visual alarms for
- sensor error or disconnected, system errors, low battery.
- low/high saturation and pulse rate.
- Automatic power-off
- Power supply:
- Two 1.5 Volt AAA batteries, rechargeable
- batteries must allow at least 2500 spot checks at 30s per spot check, or at least 12 hours of operation
- Operating conditions:
- Temperature: 5 to 40°C
- Humidity: 10 to 95%, non-condensing
- Atmospheric Pressure: 540 to 1060 mBa
Dimensions
- 7.4 x 4.1 x 3.0 cm
- Weight: 73 g
Instructions for use
Fingertips pulse oximeter are not adapted for neonate and paediatric patients.
Fingertip pulse oximeter are less accurate than the handheld oximeters and battery level of the device could have an influence on the result.
Be aware that multiple factors can affect the accuracy of a pulse oximeter reading, such as poor circulation, skin pigmentation, skin thickness, skin temperature, current tobacco use, and use of fingernail polish.
Make diagnosis and treatment decisions based on trends in pulse oximeter readings over time, rather than absolute thresholds.
Precautions for Use
These devices do not contain the necessary alarms and should not be used for continuous monitoring.
Maintenance
Clean and disinfect the fingertip oximeter after each patient tested, according to the manufacturer's instructions and IPC recommendations.
MSF requirements
A fingertip pulse oximeter allows to perform rapid assessment at triage points or when measuring vital signs at a lower cost than usual pulse oximeter/monitor.
Fingertip pulse oximeters allow to spare more advanced devices for continuous/continual monitoring of patients.





![[KMEDMSUP05E] (IEHK 2024 suppl. module) SUPPLEMENTARY EQUIPMENT UNIT](/web/image/product.template/583179/image_256/%5BKMEDMSUP05E%5D%20%28IEHK%202024%20suppl.%20module%29%20SUPPLEMENTARY%20EQUIPMENT%20UNIT?unique=8aaf2f3)