DISPENSING SPIKE, non-vented, 2-W valve+ needleless-connect.
STD
SINSTRFDS2-
Valid Article
Account code:
60210
HS Code:
901839
Last Updated on:
13/11/2025, 23:36:41
Single use
A single-use device, also referred to as a disposable device, is intended for use on one patient during a single procedure. It is not intended to be reprocessed (i.e., cleaned and disinfected or sterilized).
CE marking: declaration that the product meets EU standards for health, safety, and environmental protection. The CE marking indicates that the product may be sold freely in any part of the European Economic Area, regardless of its country of origin.
A0704 - Systems for reconstitution and administration of pharmaceuticals
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes
The product is part of at least one Kit.
A kit is a collection of products (medical and/or logistic) that are needed for a certain intervention in emergency. The choice and quantity of the articles reflects the MSF protocols for this specific situation. The use of Kits allows to start an intervention without a detailed evaluation.
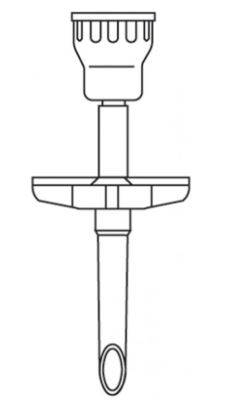
DISPENSING SPIKE with needleless connector
Definition
A sterile device designed to transfer medical fluids (e.g., drugs and solutions) between a flexible or semi-rigid container / pouch and a syringe via a needleless connector.
Specifications
Some restricted information has been hidden. Sign in
to see this information
Technical specifications
- Non vented dispensing pin
- Self-sealing valve system: 2-way valve to prevent leakage especially with hanging containers
- Needle-less injection port
- Luer activated (Luer slip compatible)
- straight fluid pathway
- easy to disinfect
- Large lumen allowing high fluid flow rate
- Sharp piercing spike permits easy penetration of rubber stopped vials and fluid containers
- With protective cap
- Sterile, for single use
Packaging & Labelling
Unit sterile packaging in peel-open pack
Instructions for use
Precautions for Use
Single use only: 1 fluid bag = 1 spike
Needle-less connector has to be disinfected with the alcoholic solution of chlorhexidine 2% before accessing it.
MSF requirements
A spike to allow repeated access to IV-bags and fluid bottles: Dispensing water for injection, electrolyte solutions etc.
The non-vented dispensing pin is the best adapted to the MSF standard IV fluid bags.
Some restricted information has been hidden. Sign in
to see this information







![[DEXTCHLHA2W] CHLORHEXIDINE 2%, 70% isopropyl alcohol, SWAB/WIPE](/web/image/product.template/570781/image_256/%5BDEXTCHLHA2W%5D%20CHLORHEXIDINE%202%25%2C%2070%25%20isopropyl%20alcohol%2C%20SWAB-WIPE?unique=8dd7c03)
![[KMEDMNUTI33] (module nut. inpatient) MEDICAL SUPPLIES 2021](/web/image/product.template/575289/image_256/%5BKMEDMNUTI33%5D%20%28module%20nut.%20inpatient%29%20MEDICAL%20SUPPLIES%202021?unique=ce93f30)
![[KMEDMNUTO33] (module nut. outpatient) MEDICAL SUPPLIES 2021](/web/image/product.template/575294/image_256/%5BKMEDMNUTO33%5D%20%28module%20nut.%20outpatient%29%20MEDICAL%20SUPPLIES%202021?unique=5c1c774)
![[KMEDMHIS24-] (mod ICU) INJECTION SUPPLIES 2021](/web/image/product.template/574424/image_256/%5BKMEDMHIS24-%5D%20%28mod%20ICU%29%20INJECTION%20SUPPLIES%202021?unique=aecada5)
![[KMEDMHHS24-] (mod hospital) INJECTION SUPPLIES](/web/image/product.template/572826/image_256/%5BKMEDMHHS24-%5D%20%28mod%20hospital%29%20INJECTION%20SUPPLIES?unique=62dc65c)