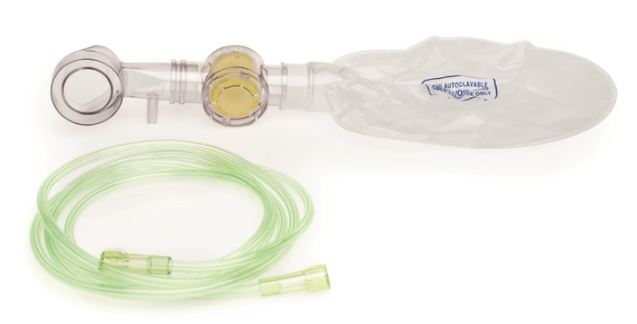
(resuscitator Laerdal Upright) OXYGEN KIT complete 846151
Valid Article
(resuscitator Laerdal Upright) OXYGEN KIT
Definition
Laerdal Upright Resuscitator (”Upright”) is a self-inflating, manual, reusable resuscitator intended for newborns and infants up to 10 kg body mass who require respiratory support. Upright can provide supplemental oxygen only when used with the accessory Oxygen Kit.
Specifications
Quality standards
Technical specifications
Delivered Oxygen Concentration Measured under room temperature conditions:
20 ml tidal volume @ 60 breaths / min | 150 ml tidal volume @ 25 breaths / min | |||
| oxygen flow (l/min) | no reservoir | with reservoir | no reservoir | with reservoir |
| 1 | 40% | 46% | 52% | 71% |
| 2 | 52% | 67% | 63% | 92% |
| 3 | 58% | 89% | 73% | 96% |
Instructions for use
Upright's pressure release valve can be operated as normal when the Oxygen Kit is attached. If any components are loose, then tighten or reassemble the device and test according protocol.
Precautions for Use
If any of the above tests fail: Disassemble, inspect the components, reassemble and repeat the complete “Testing before use” procedure
Maintenance
The Oxygen Kit may be reused provided reprocessing procedures are followed between each patient use. The kit (except for the Oxygen Reservoir and the Oxygen Tube) must be cleaned and disinfected before first use.



![[KMEDMHHE22-] (mod hospital) BASIC RESUSCITATION EQUIPMENT](/web/image/product.template/572769/image_256/%5BKMEDMHHE22-%5D%20%28mod%20hospital%29%20BASIC%20RESUSCITATION%20EQUIPMENT?unique=a9e1038)
![[KMEDMHIE21-] (mod ICU) EXAMINATION-RESUSCITATION EQUIPMENT](/web/image/product.template/574340/image_256/%5BKMEDMHIE21-%5D%20%28mod%20ICU%29%20EXAMINATION-RESUSCITATION%20EQUIPMENT?unique=22350c3)
![[KMEDMHOE17-] (mod OT Room) ANESTHESIA-RESUSCITATION EQ.](/web/image/product.template/572539/image_256/%5BKMEDMHOE17-%5D%20%28mod%20OT%20Room%29%20ANESTHESIA-RESUSCITATION%20EQ.?unique=8f85d22)
![[KMEDMHDE31-] (mod delivery & neonate) MEDICAL EQUIPMENT 2021](/web/image/product.template/574359/image_256/%5BKMEDMHDE31-%5D%20%28mod%20delivery%20%26%20neonate%29%20MEDICAL%20EQUIPMENT%202021?unique=de395b9)