(blds. syst.) SAFETY TUBE HOLDER, retractable, semi-aut,s.u.
Valid Article
(blood sampling) SAFETY TUBE HOLDER, retractable
Definition
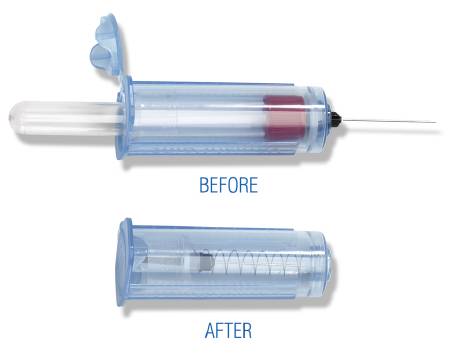
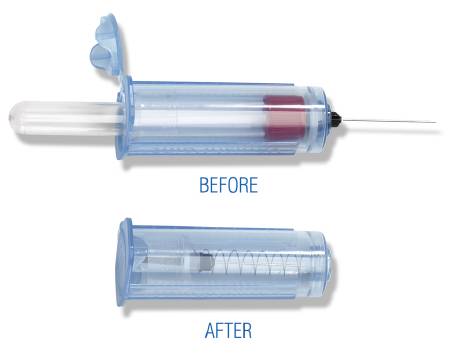
A non-sterile, hand-held cylindrical device designed to be used together with a vacuum blood collection tube to draw blood samples from a patient. The device features an integrated active safety mechanism.
It is a hollow plastic adaptor to which the user attaches a blood collection needle at one end and into which the blood collection tube is inserted at the other end; this allows a multiple-tube blood collection via one venipuncture. The semi-automatic activation of the safety mechanism by the user retracts the needle into the holder protecting the needle completely making it safe to dispose after use.
Specifications
Technical specifications
- Plastic tube holder, transparent
- Compatible with standard blood sampling needles with thread, length up to 38 mm and regular Vacutainer tubes
- Single use device
- Non sterile
- Actif retractable safety system, integrated in the device and protecting the needle completely after activation.
- One-handed activation by the clinician after withdrawal of the last blood tube by a semi-automatic system closing the lid at the rear end
Packaging & Labelling
250 units / box
Instructions for use
Please consult the “Manual of nursing Care Procedures, MSF, 2020” available online via the Nursing care working Group sharepoint page.
MSF requirements
- No technique change, ease of use
- Compatible with the standard blood sampling needles and tubes (Vacutainer)
- Retractable system covering the whole needle after activation was selected as safety system for high risk context (VHF)

![[KMEDMHMI22-] MODULE, VHF INVESTIGATION 2 persons/10 samples 2021](/web/image/product.template/574349/image_256/%5BKMEDMHMI22-%5D%20MODULE%2C%20VHF%20INVESTIGATION%202%20persons-10%20samples%202021?unique=66a6919)
![[KMEDMEBO05A] (module VHF) SAMPLING](/web/image/product.template/571740/image_256/%5BKMEDMEBO05A%5D%20%28module%20VHF%29%20SAMPLING?unique=d43f4a9)
![[KMEDMEBO17A] (module VHF) SAMPLING AND SMALL ISOLATION](/web/image/product.template/571758/image_256/%5BKMEDMEBO17A%5D%20%28module%20VHF%29%20SAMPLING%20AND%20SMALL%20ISOLATION?unique=6e19745)