ELECTRONIC SPHYGMOMANOMETER, manual inflation(Microlife VSA)
Valid Article
ELECTRONIC SPHYGMOMANOMETER, Microlife VSA
Definition
Handheld semi-automatic oscillometric device to measure blood pressure in people aged 12 years or older.
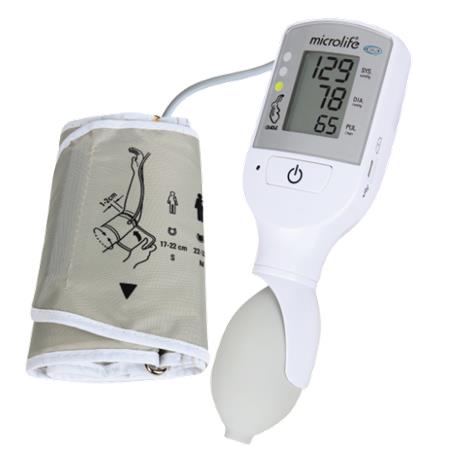
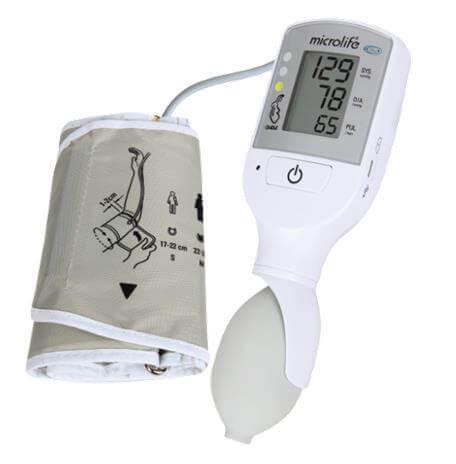
An electronic device, typically digital, designed to noninvasively measure blood pressure using a manually-inflated cuff that fits around the arm. It displays current heart rate in addition to systolic and diastolic blood pressures; it has memory to store blood pressure values and a visual warning system if blood pressure exceeds pre-set limits. This device is designed to be portable (e.g., hand-held or carried in a pouch).
Synonym
Vital Signs Alert - CRADLE device
Vital Signs Monitor (Cradle) for pregnant women
Specifications
The device incorporates a traffic light early warning system, aiming to alert in a visual way (color code)to abnormalities in vital signs secondary to pre-eclampsia or shock (due to bleeding or sepsis). It is suitable for use in low-resource settings according to WHO criteria.
- measuring unit:
- ON/OFF button
- LCD-display
- cuff socket
- battery compartment
- Micro USB port (for charging only)
- traffic light
- charging indicators
- cuff: the VSA device comes with a medium cuff as standard
- cuff connector
- pump ball: for manual inflation of the cuff
Technical specifications
- Weight: 157.7 g (including battery)
- Dimension: 167 x 60 x 41 mm
- Display: LCD-Display (Liquid Crystal Display)
- Measuring procedure: oscillometric, corresponding to Korotkoff method Phase I systolic, Phase V diastolic.
- measurement range: 30-280 mmHg blood pressure
- 40-200 beats per minute-pulse
- cuff pressure display range: 0-299 mmHg
- Resolution: 1 mmHg
- Static accuracy: pressure within ± 3 mmHg
- Pulse accuracy: ± 5% of the readout value
- Voltage source: specify battery type (3.7V, 350 mAh), rechargeable via standard MicroUSB
- Battery life: approx. 200 measurements per recharge cycle
Supplied with the Article
- Cuff size M for circumference of upper arm 22 - 32 cm
- Battery type 3.7V, 350 mAh, rechargeable via standard MicroUSB
- USB cable
To be Ordered Separately
Cuff size L for circumference of upper arm 32 - 42 cm
Instructions for use
Read carefully the user manual
Maintenance
- Device care: Clean the device only with a soft, dry cloth
- Cleaning the cuff: take out the bladder. Clean the cuff with warm water and soap detergent. Air dry.
Storage
Do not expose the device to either extreme temperatures,
humidity, dust or direct sunlight.
- Storage conditions: -20 - +55 °C; 15 - 90 % relative maximum humidity
- Operation conditions: 10 - 40 °C; 15 - 90 % relative maximum humidity
MSF requirements
Meets the World Health Organisation (WHO) criteria for devices suitable for in low-resource settings
It can be used with USB phone chargers.
Requires minimal training.

![[EMEQSPHY5CL] (sphygmomanometer Microlife) CUFF adult L 32-42cm](/web/image/product.template/577974/image_256/%5BEMEQSPHY5CL%5D%20%28sphygmomanometer%20Microlife%29%20CUFF%20adult%20L%2032-42cm?unique=ebe9aac)