VACUUM EXTRACTOR obstetrical (Kiwi OmniCup), manual s.u.
Valid Article
VACUUM EXTRACTOR obstetrical (Kiwi OmniCup), manual s.u.
Definition
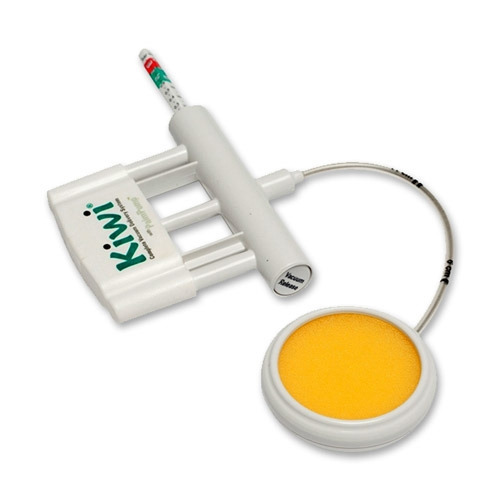
An assembly of hand-operated devices designed to facilitate the delivery of a foetus during vaginal childbirth by enabling traction to be applied to the foetal head by means of a suction cup that is attached to the scalp. It consists of a cup with a soft inner liner to prevent tissue damage, a hand-operated pump, and a handle used to apply traction. It is typically used for dystocia, uterine inertia, maternal exhaustion, maternal/foetal distress. This is a single-use device.
Specifications
Technical specifications
PalmPump
- simple hand vacuum pump
- thumb or finger activated vacuum release valve
- accurate vacuum indicator.
The Kiwi OmniCup
- a Malmstrom-style cup = rigid type with a firm flattened mushroom-shaped cup and circular ridge around the cup rim
- universal cup for all presentations
- low profile: 50mm Ø x 20mm
- with a handle grip for easy insertion = assists with proper placement in fetal malpresentations
- flexible stem (length 15 cm) enables the cup to be placed over the flexion point no matter the position of the fetal head
Sterile, for single use
Instructions for use
Precautions for Use
If traction is misdirected or too forceful, vacuum may be broken. Before replacing cup, examine fetal scalp for trauma and re-assess presentation and position.
As with forceps procedures, there should be a willingness to abandon attempts at vacuum delivery if satisfactory progress is not made.
MSF requirements
The disposable device replaces the reusable one as there were several quality problems notified.


![[ESURVENT1S-] VACUUM EXTRACTOR, obstetrical, hand-op., 40-50-60 mm + PARTS](/web/image/product.template/570277/image_256/%5BESURVENT1S-%5D%20VACUUM%20EXTRACTOR%2C%20obstetrical%2C%20hand-op.%2C%2040-50-60%20mm%20%2B%20PARTS?unique=fb9a656)
![[KMEDMHHE19B] (mod hospital) GYNAECO/OBSTETRIC MATERIAL VERSION B](/web/image/product.template/572757/image_256/%5BKMEDMHHE19B%5D%20%28mod%20hospital%29%20GYNAECO-OBSTETRIC%20MATERIAL%20VERSION%20B?unique=49d2a38)