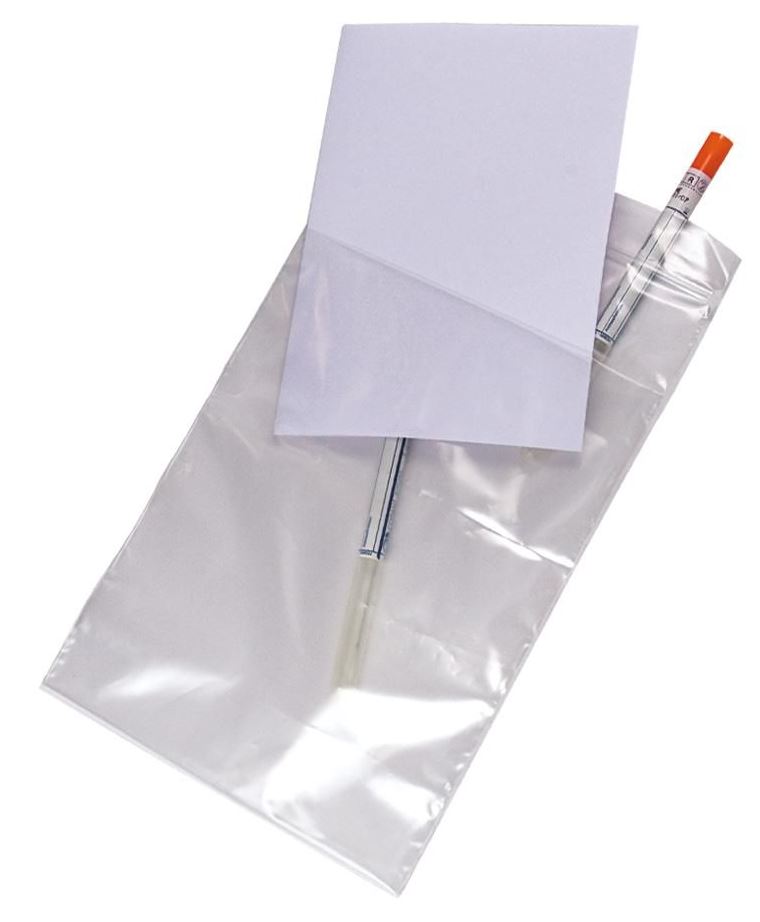
POUCH, with pocket for documents, polyethylene, 18x27cm
STD
STSSPOUPD1-
Valid Article
HS Code:
392390
Last Updated on:
07/11/2024, 18:40:16
Single use
A single-use device, also referred to as a disposable device, is intended for use on one patient during a single procedure. It is not intended to be reprocessed (i.e., cleaned and disinfected or sterilized).
W05020101 - Samples transport, pouches
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
POUCH, with pocket for documents, polyethylene
Definition
Disposable transparent pouch for sample transport.
Specifications
Technical specifications
- Transparent pouch
- Material: polyethylene
- Leak resistant
- Size: +/- 20 x 30 cm
- Zipper seal allows easy re-closure or other closing system
- Optional: large white marking strip for write-on allows easy identification of samples
Instructions for use
Some restricted information has been hidden. Sign in
to see this information
MSF requirements
Reserved for projects using the Mini-Lab
Some restricted information has been hidden. Sign in
to see this information




![[KMEDKBLB02-] BACTERIOLOGY LABORATORY KIT, Mini-Lab STARTERKIT 800 samples](/web/image/product.template/579040/image_256/%5BKMEDKBLB02-%5D%20BACTERIOLOGY%20LABORATORY%20KIT%2C%20Mini-Lab%20STARTERKIT%20800%20samples?unique=3665ce3)