URINE FLOWMETER, grad. 400ml overflow 2l drain. bag, ster.
STD
SCTDBAGUF42
Valid Article
Account code:
60210
HS Code:
901890
Last Updated on:
07/11/2024, 20:50:54
Single use
A single-use device, also referred to as a disposable device, is intended for use on one patient during a single procedure. It is not intended to be reprocessed (i.e., cleaned and disinfected or sterilized).
A06030302 - Urine flowmeter sets
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes
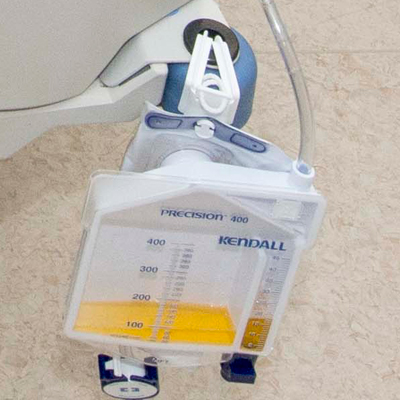
URINE FLOWMETER overflow system
Definition
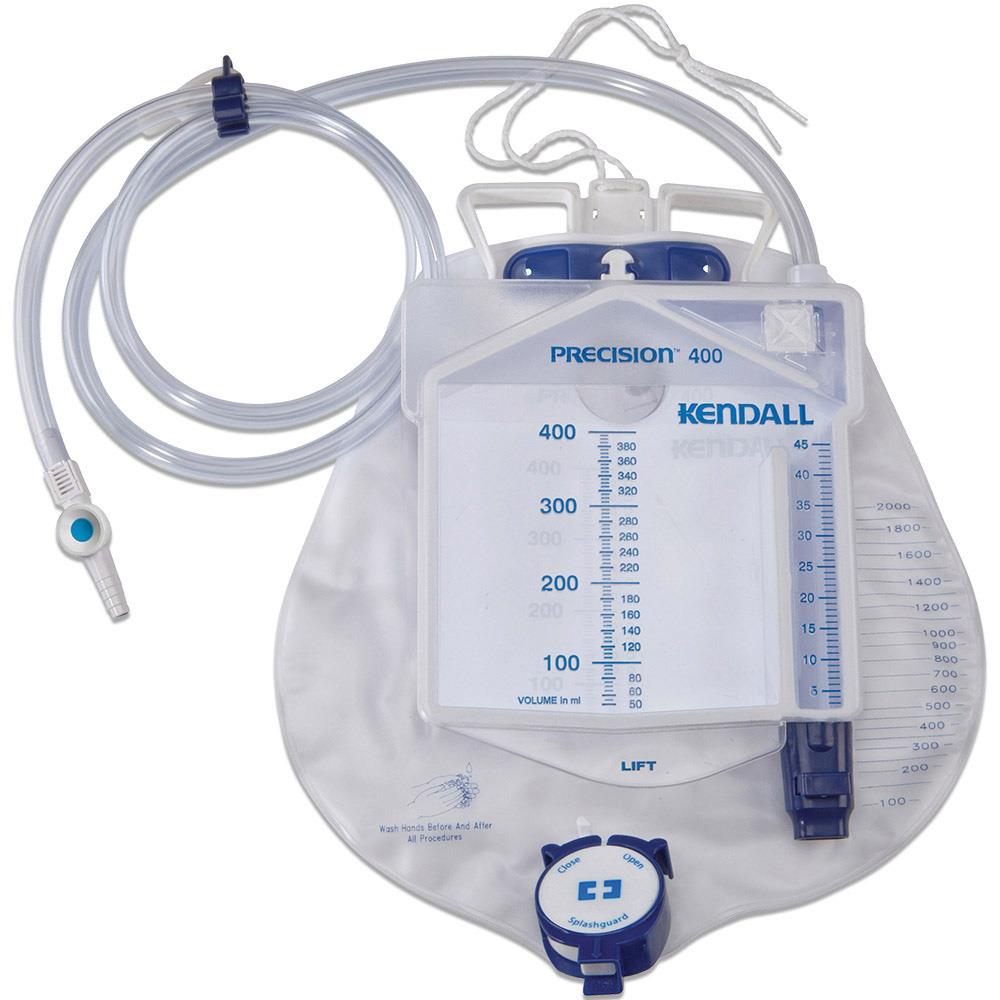
A sterile device that is used to measure precisely the urine output of a catheterized patient. The device includes a graduated drip chamber which can be emptied into a drainable 2L bag with an overflow system. It is used for patients requiring close monitoring of urine output on an hourly basis.
Specifications
Quality standards
ISO 8669-2, 1996, edition 2, (confirmed 2023) Urine collection bags - Part 2: Requirements and test methods
Technical specifications
- Precision urimeter
- rigid container
- capacity 400 ml
- graduations: 1 ml for the first 45 ml, per 5 ml for the remaining 400 ml
- no-return system
- automatic overflow towards the urine bag
- Bag:
- flexible plastic, transparent frontside
- capacity: 2 litres
- graduations every 50 ml up to 500 ml, then per 100 ml up to 2l
- non-return valve at the tube inlet
- emptying system, single handed manipulation
- integrated hanging hook
- Drainage tube:
- flexible plastic
- length: ± 150 cm
- diameter: 8 mm
- universal connector for connecting to a catheter
- needle-free sampling port to be used with a Luer syringe
- protective cap
- Inside AND outside sterile device, for single use
Packaging & Labelling
Unit sterile packaging in peel-open pack
MSF requirements
Hourly urine output is largely admitted to be an important monitoring parameter in severely ill patients in many settings including: OTs, ICUs, Emergency departments PICU/NICU, burn units.
Hourly urine output values are often recommended as a target for IV fluid and vasopressor therapy in states of shock.
Some restricted information has been hidden. Sign in
to see this information

![[KMEDMHIS25-] (mod ICU) CATHETERS, TUBES and DRAINS 2021](/web/image/product.template/574344/image_256/%5BKMEDMHIS25-%5D%20%28mod%20ICU%29%20CATHETERS%2C%20TUBES%20and%20DRAINS%202021?unique=d9788e5)
![[SCTDBAGU2VSS] URINE BAG 2 l, drain., non-ret. valves, sampling port, ster.](/web/image/product.template/571478/image_256/%5BSCTDBAGU2VSS%5D%20URINE%20BAG%202%20l%2C%20drain.%2C%20non-ret.%20valves%2C%20sampling%20port%2C%20ster.?unique=130f32a)