PATIENT MONITORING SYSTEM + acc (Philips MX400 + MMS X3)
Valid Article
PATIENT MONITORING SYSTEM + acc (Philips MX400 + MMS X3)
The new model replaces the EEMDMONE8--PATIENT MONITORING SYSTEM + acc (Philips MX400 + MMS X2) because the extension MMS x2 has been discontinued and replaced by MMS x3.
Definition
An assembly of devices designed for continuous assessment of several vital physiologic parameters (e.g., ECG, blood pressure, heart rate, temperature, cardiac output, apnoea, and respiratory/anaesthetic gas concentrations) of one patient. It typically includes a central station monitor that receives, consolidates, and displays the information, and a bedside patient monitor; it often includes portable radio transmitters, receivers, and antennas (telemetry systems) to allow monitoring of an ambulatory patient; it is not dedicated to neonatal or ambulatory use. The system is used to evaluate and observe trends in a compromised or unstable patient in intensive or general healthcare settings.
GMDN : Single-patient physiologic monitoring system (33586)
Specifications
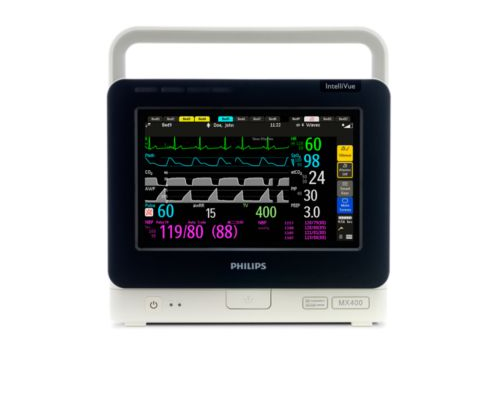
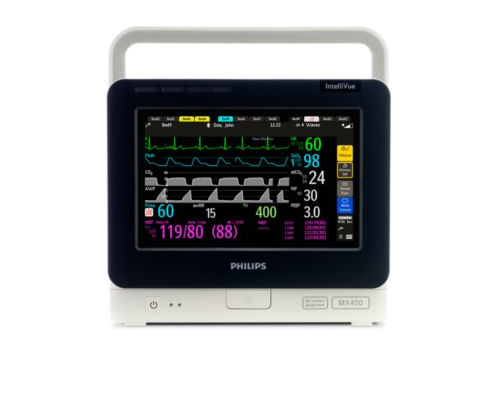
The MX400 provides monitoring in a compact, transportable unit and designed to monitor a wide range of vital signs.
The MMS X3 is a transport monitor that's also a measurement module, offering continuity of patient data and transport across all levels of patient monitoring.
Technical specifications
MX400:
- 9"-wide touchscreen
- The display automatically adjusts screen brightness
- Built-in handle and battery operation
- Measured parameters:
- NIBP,
- SpO2,
- TEMP,
- RESP,
- ECG
MMS X
- additional battery run time of up to six hours (11 Wh) for intra-hospital patient transport and concurrent CO₂ measurement with additional invasive blood pressure and temperature measurement.
- colorful touchscreen with 3.5" display
- Operating Voltage: 36 to 60 V DC floating
- Cardiotach
- Range
- Adult/pedi: 15 to 300 bpm
- Neo range: 15 to 350 bpm
- PVC Rate
- Range: 0 to 300 bpm
- ST Numeric
- Range: -20 to +20 mm
- QT Numeric
- Range: 200 to 800 ms
- QTc Numeric
- Range: 200 to 800 ms
- ΔQTc Numeric
- Range: -600 to +600 ms
- QT-HR Numeric
- Range - adult: 15 to 300 bpm
- Range - pediatric and neonatal: 15 to 350 bpm
- Sinus and SV Rhythm Ranges
- Brady
- Adult: 15 to 59 bpm
- Pedi: 15 to 79 bpm
- Neo: 15 to 89 bpm
- Normal
- Adult: 60 to 100 bpm
- Pedi: 80 to 160 bpm
- Neo: 90 to 180 bpm
- Tachy
- Adult: >100 bpm
- Pedi: >160 bpm
- Neo: >180 bpm
Dimensions
MMS X2:
- Height: 9.9 cm
- Width: 18.8 cm
- Depth: 8.6 cm
- Weight: 1.25 kg
Transport Dangerous Goods
- UN3481 Lithium ion batteries contained in equipment
- Class: 9
- Packing Instructions: 967 Section II
- The label must include the UN code of the battery + an emergency number to contact in case of an incident
- Limit per package:
- Pax A/C (Passengers & Cargo Aircraft) = 5 kg
- CAO (Cargo Aircraft Only) = 5 kg
Instructions for use
Precautions for Use
- Prohibited Environments: The monitors are not intended for use in an MRI environment or in an oxygen-enriched environment (for example, hyperbaric chambers).
- Grounding: Remember that to avoid the risk of electric shock, the monitor must be grounded during operation. If a three-wire receptacle is not available, consult the hospital electrician. Never use a three-wire to twowire adapter.
- Explosion Hazard: Do not use in the presence of flammable anesthetics or gases, such as a flammable anesthetic mixture with air, oxygen or nitrous oxide. Use of the devices in such an environment may present an explosion hazard.
- Reuse: Never reuse disposable transducers, sensors, accessories and so forth that are intended for single use, or single patient use only. Reuse may compromise device functionality and system performance and cause a potential hazard.
MSF requirements
Restricted to Ebola intervention
Description approved by OCBA referent (Alicia Aglio) in July 2023.

![[EEMDMONA801] (Philips, MX400 + MMS X2/X3) CABLE FOR INTELLIVUE M8022A](/web/image/product.template/581176/image_256/%5BEEMDMONA801%5D%20%28Philips%2C%20MX400%20%2B%20MMS%20X2-X3%29%20CABLE%20FOR%20INTELLIVUE%20M8022A?unique=1f49c0c)
![[EEMDMONA802] (Philips, MX400 + MMS X2/X3) SYSTEM CABLE - 25m SC9](/web/image/product.template/581172/image_256/%5BEEMDMONA802%5D%20%28Philips%2C%20MX400%20%2B%20MMS%20X2-X3%29%20SYSTEM%20CABLE%20-%2025m%20SC9?unique=fed1621)
![[EEMDMONA803] (Philips MX400+MMS X2/X3) ECG CABLE 3 wires, AAMI/CEI, 2.7 m](/web/image/product.template/581180/image_256/%5BEEMDMONA803%5D%20%28Philips%20MX400%2BMMS%20X2-X3%29%20ECG%20CABLE%203%20wires%2C%20AAMI-CEI%2C%202.7%20m?unique=0f174e3)
![[EEMDMONA805] (Philips, MX400 + MMS X2/X3) NIBP CUFF TUBING ad 3m](/web/image/product.template/581185/image_256/%5BEEMDMONA805%5D%20%28Philips%2C%20MX400%20%2B%20MMS%20X2-X3%29%20NIBP%20CUFF%20TUBING%20ad%203m?unique=e71d637)
![[EEMDMONA806] (Philips MX400+MMS X2/X3) CONNECTING CABLE TEMP. PROBE](/web/image/product.template/581179/image_256/%5BEEMDMONA806%5D%20%28Philips%20MX400%2BMMS%20X2-X3%29%20CONNECTING%20CABLE%20TEMP.%20PROBE?unique=0f174e3)
![[EEMDMONA807] (Philips, MX400 + MMS X3) PATIENT CABLE LNCS, LNC-10 3345](/web/image/product.template/581178/image_256/%5BEEMDMONA807%5D%20%28Philips%2C%20MX400%20%2B%20MMS%20X3%29%20PATIENT%20CABLE%20LNCS%2C%20LNC-10%203345?unique=9f9af86)
![[EEMDMONA808] (Philips, MX400 + MMS X2/X3) SYSTEM CABLE 10m M8022A SC6](/web/image/product.template/581174/image_256/%5BEEMDMONA808%5D%20%28Philips%2C%20MX400%20%2B%20MMS%20X2-X3%29%20SYSTEM%20CABLE%2010m%20M8022A%20SC6?unique=85479bb)
![[EEMDMONC801] (Philips, MX400 + MMS X2/X3) CUFF single patient, ped.](/web/image/product.template/581182/image_256/%5BEEMDMONC801%5D%20%28Philips%2C%20MX400%20%2B%20MMS%20X2-X3%29%20CUFF%20single%20patient%2C%20ped.?unique=0f174e3)
![[EEMDMONC802] (Philips, MX400 + MMS X2/X3) CUFF single patient, small adt.](/web/image/product.template/581183/image_256/%5BEEMDMONC802%5D%20%28Philips%2C%20MX400%20%2B%20MMS%20X2-X3%29%20CUFF%20single%20patient%2C%20small%20adt.?unique=0f174e3)
![[EEMDMONC803] (Philips, MX400 + MMS X2/X3) CUFF single patient, adult](/web/image/product.template/581181/image_256/%5BEEMDMONC803%5D%20%28Philips%2C%20MX400%20%2B%20MMS%20X2-X3%29%20CUFF%20single%20patient%2C%20adult?unique=0f174e3)
![[EEMDMONC804] (Philips, MX400 + MMS X2/X3) SKIN TEMPERATURE PROBE, u.u.](/web/image/product.template/581177/image_256/%5BEEMDMONC804%5D%20%28Philips%2C%20MX400%20%2B%20MMS%20X2-X3%29%20SKIN%20TEMPERATURE%20PROBE%2C%20u.u.?unique=0f174e3)
![[EEMDMONC805] (Philips, MX400 + MMS X2/X3) THERMAL PAPER](/web/image/product.template/581186/image_256/%5BEEMDMONC805%5D%20%28Philips%2C%20MX400%20%2B%20MMS%20X2-X3%29%20THERMAL%20PAPER?unique=0f174e3)
![[EEMDMONC808] (Philips MX400+MMS X2/X3) SET OF 3 WIRES u.u., bedside, IEC](/web/image/product.template/581187/image_256/%5BEEMDMONC808%5D%20%28Philips%20MX400%2BMMS%20X2-X3%29%20SET%20OF%203%20WIRES%20u.u.%2C%20bedside%2C%20IEC?unique=0f174e3)
![[EEMDMONE81-] (Philips MX400+MMS X2/X3) PATIENT MONITOR, IntelliVue MX400](/web/image/product.template/581173/image_256/%5BEEMDMONE81-%5D%20%28Philips%20MX400%2BMMS%20X2-X3%29%20PATIENT%20MONITOR%2C%20IntelliVue%20MX400?unique=f4c5616)
![[EEMDMONE83-] (Philips, MX400 + MMS X3) MULTI-MEASUREMENT MODULE Intell X3](/web/image/product.template/581175/image_256/%5BEEMDMONE83-%5D%20%28Philips%2C%20MX400%20%2B%20MMS%20X3%29%20MULTI-MEASUREMENT%20MODULE%20Intell%20X3?unique=f4c5616)
![[EEMDMONS801] (Philips, MX400) BATTERY Li-Ion M4605A](/web/image/product.template/581171/image_256/%5BEEMDMONS801%5D%20%28Philips%2C%20MX400%29%20BATTERY%20Li-Ion%20M4605A?unique=85479bb)