PELVIC BINDER, medium (SAM Sling II)
Valid Article
PELVIC BINDER
Definition
A belt-like support designed to be wrapped tightly around the pelvis (around the greater trochanters) of a patient suspected of a pelvic fracture to support the pelvis during transportation. It is typically intended to be used in an emergency setting to stabilize pelvic fractures. This is a reusable device.
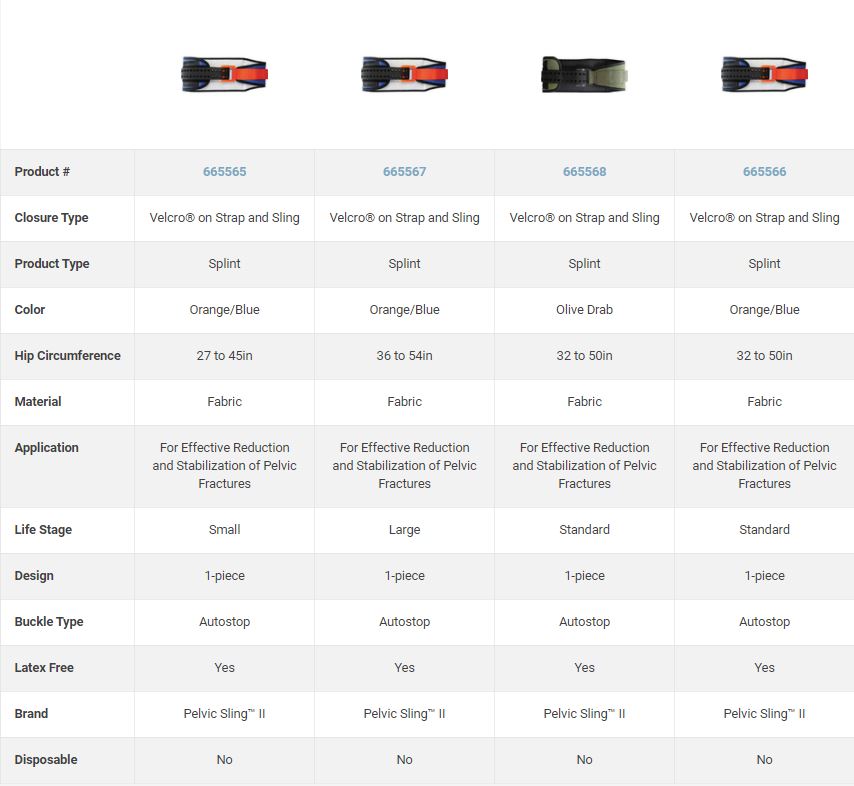
Specifications
Technical specifications
- Radiolucent (except the buckles' springs)
- Velcro strap to adjust width
- Autostop buckle to avoid over- or under tightening of the sling. Once optimal compressive force is reached, two prongs activate, clicking to confirm correct application.
- Standard size: 81-127 cm
- Reusable
Instructions for use
This technique is properly done with 2 people.
A correctly placed pelvic binder should allow
- complete exposure of the abdomen and therefore must not interfere in case a laparotomy is needed,
- as well access to the genital area.
The pelvic binder is placed in the same way in pregnant women.
Steps with the SAM Pelvic sling II:
- Slide the binder under the patient's knees.
- IDENTIFY THE GREATER TROCANTERS (GT): they are felt as bony prominences laterally on the proximal thigh, they are considerably inferior than the iliac crests.
- Slide the binder from below the knees until it's under the hand placed at the GT level.
- Simultaneously, both operators will slightly lift the pelvis allowing space to slide the binder.
- Secure the binder.
Precautions for Use
If the binder is to be applied for extended periods, the skin should be inspected at regular intervals. Be especially observant when massive fluid resuscitation is required. Under these conditions, the binder may have to be periodically released to accommodate increased pelvic volume.
If the pelvic fracture has been ruled out, the binder must be removed. The pelvic binder should be released very slowly.
Remark: a correctly placed binder can mimic a normal image of a pelvis x-ray, therefore, if the patient is hemodynamically stable, the binder must be loosened up (NOT REMOVED) and the x ray repeated.
The use of a pelvic binder on children is not recommended (lack of data).
Maintenance
Do NOT autoclave the pelvic binder.
Can be cleaned by hand washing with standard detergent.
Can be cleaned and disinfected with a detergent/disinfectant for surfaces/non-invasive medical equipment. Prepare use-solution in concentration required. Always read the label and product information before use. Do not mix with other products. Make sure to wet the surfaces completely, use a wipe or towel, keep them wet for the whole exposure time.
MSF requirements
Pelvic belt to be used in suspected /confirmed pelvic fracture to reduce bleeding and mortality related to it.
This is a standard device in all trauma centres level 1.








![[KMEDMHAE23-] (mod AMP) MEDICAL EQUIPMENT 2021](/web/image/product.template/574351/image_256/%5BKMEDMHAE23-%5D%20%28mod%20AMP%29%20MEDICAL%20EQUIPMENT%202021?unique=bf82f3a)
![[KMEDMHEE321A] (mod emergency) COMPLEMENTARY RESUSCITATION EQUIPMENT 2021](/web/image/product.template/574367/image_256/%5BKMEDMHEE321A%5D%20%28mod%20emergency%29%20COMPLEMENTARY%20RESUSCITATION%20EQUIPMENT%202021?unique=d19c638)