TUBE UTM VIRUS 3ml + 2 SWABS, flocked tip, plastic stick
STD
STSSTRTUUTM3
Valid Article
Account code:
60200
Last Updated on:
03/11/2022, 15:50:08
A1102 - Sample collection swabs with transport medium
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
Thermosensitive codes are defined for storage and transportation temperature requirements of the products.
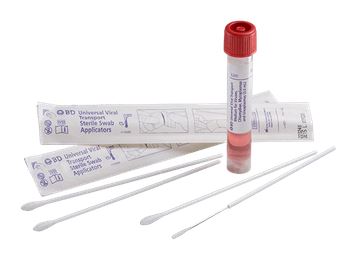
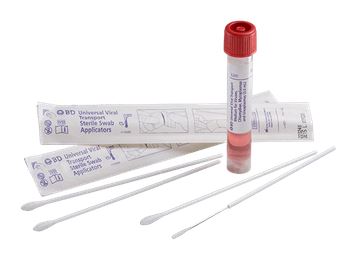
UNIVERSAL & VIRAL TRANSPORT MEDIUM + swab
Definition
Kit containing Universal / Viral Transport Medium and 2 swabs for the collection and transport of specimens containing viruses, chlamydiae, mycoplasmas or ureaplasmas from the collection site to the testing laboratory.
Specifications
- STSSTRTUUTM3- (1st choice)
- 3 ml of UTM-RT® medium in 16x100 mm screw-cap tube with internal shaped conical bottom
- One minitip size plastic applicator swab with flocked nylon fiber with breaking point
- One regular size plastic applicator swab with flocked nylon fiber tip with breaking point
- STSSTRTTUUTM1- (2nd choice)
- 3 ml of UTM medium in screw-cap tube
- Two swabs with synthetic tip and plastic handle with breaking point
Caution
In the exceptional situation that the references above are not available, please contact your OC laboratory referent to identify an alternative. All specifications below should be met:
- Swabs
- Swab with polysester/Rayon/Nylon tip, ideally flocked (not cotton!)
- 2 swabs/patient
- Plastic stick/handle
- Sterile
- Viral transport medium (1/patient)
- 1,2 or 3 ml Viral transport medium isotonic to mammalian host cells, supplemented with antibiotics to inhibit growth of competing bacteria and yeast.
- Should not contain Guanidine Thiocyanate (= toxic)
Both to comply with CLSI standard M40-A
Some restricted information has been hidden. Sign in
to see this information
Technical specifications
- Medium Composition
- Hanks' Balanced Salts
- bovine serum albumin
- L-cysteine, gelatin
- sucrose
- L-glutamic acid
- HEPES buffer
- vancomycin
- amphotericin B
- colistin
- phenol red
- Mini-tip swabs are suitable for nasopharyngeal sample collection
Packaging
Packaging: 50 kits/package - 6 x 50 kits/carton
Instructions for use
Some restricted information has been hidden. Sign in
to see this information
Storage
- Temperature between 2 and 25°C.
- Do not overheat or freeze prior to use
- The product must be stored in its original packaging
- Shelf life: 16 months
MSF requirements
Sample collection and storage of the sample during transport to the laboratory, for suspiscions of viruses, chlamydiae, mycoplasmas or ureaplasmas
Some restricted information has been hidden. Sign in
to see this information