HIGH-FLOW HEATED RESPIRATORY HUMIDIFIER (AIRVO 2)
Valid Article
HIGH-FLOW HEATED RESPIRATORY HUMIDIFIER (AIRVO 2)
Definition
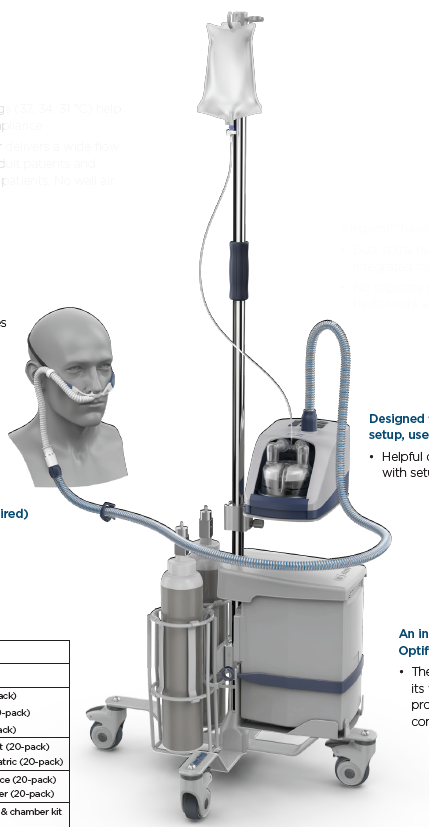
A mains electricity (AC-powered) device designed for the treatment of spontaneously breathing patients who would benefit from receiving high-flow (typically greater than 15 L/min) heated and humidified ambient air or an air/oxygen mixtures. The gases are delivered to the patient's airway via the nose/mouth or a tracheostoma.
It typically consists of a gas flow generator, a heating element, humidification chamber, and breathing tube(s).
Specifications
Components
- Tube and chamber kit
- Auto-fill water chamber MR290
- Heated breathing tube
- Interface adaptors
- tracheostomy direct connection OPT870
- mask interface adaptor 22 mm RT013
- Hospital stand 900PT42
- Pole mounting tray 900PT405
- Oxygen inlet extension kit 900PT422
- Filter holder 900PT912
- Air filter 900PT913
Technical specifications
- Supply Frequency: 50/60 Hz
- Supply Voltage: 230 V~ 1.8 A
- Supply Current: 1.0 A max at 230 V~1.8 A
- Heater Plate: 150 W
- Heater Wire:22 V~, 2.73 A, 60 W , 50/60 Hz
- Heater Plate Over-temperature Cutout: 118 ± 6 °C
- Sound pressure level: Alarms exceed 45dbA @ 1 m
- Auditory alarm pause 115 seconds
- Humidification performances
- >33 mg/l at 27°C target
- >12mg/l at 34°C target
- >12mg/l at 31°C target
- Maximum temperature of delivered gas : 43°C (109°F)
- Maximum flow range: 10-60 l/min (default); 2-25 l/min (Junior mode)
- Maximum oxygen input: 60l/min
- Warm-up time:
- 10 minutes to 31 °C (88 °F),
- 30 minutes to 37 °C (98.6 °F)
- using a MR290 chamber with flow rate of 35 L/min and starting temperature 23 ± 2 °C (73 ± 3 °F)
- Oxygen analyzer accuracy: < ± 4% of gas level) within the range 25-95% O2
- Operating conditions:
- 18-28 °C (64-82 °F),
- 10-95% RH
- Altitude 0 to 2000 m (6000 feet)
- Protection against electric shock
- Type B
- Class II double insulated
Dimensions
- 295 mm x 170 mm x 175 mm (11.6 in x 6.7 in x 6.9 in)
- Weight:
- 2.2 kg (4.8 lb) unit only,
- 3.4 kg (7.5 lb) packaged in bag incl. accessories
To be Ordered Separately
Patient interface:
- nasal cannula: infant, paediatric, adult S/M/L
- oxygen mask
- tracheostomy tube
Instructions for use
The input of this flowmeter is French standard NF and the wall outlet will need to compatible, otherwise an adapter will be required. Contact your OC Biomed for clarification
MSF requirements
For the treatment of spontaneously breathing patients who would benefit from receiving high flow warmed and humidified respiratory gases, in the Coronavirus epidemics.
Description approved by referents of OCP (Ribhar Ndouba) and OCB (Jonathan Delchambre) in August 2022.


![[EEMDHRHA201] (hum Airvo2) FLOWMETER, OXYGEN, 0-70l/min](/web/image/product.template/579117/image_256/%5BEEMDHRHA201%5D%20%28hum%20Airvo2%29%20FLOWMETER%2C%20OXYGEN%2C%200-70l-min?unique=3e30a3a)
![[EEMDHRHA202] (hum Airvo2) FIXING PLATE 900PT405](/web/image/product.template/579145/image_256/%5BEEMDHRHA202%5D%20%28hum%20Airvo2%29%20FIXING%20PLATE%20900PT405?unique=712437e)
![[EEMDHRHA203] (hum Airvo2) HOSPITAL POLE STAND 900PT421](/web/image/product.template/579144/image_256/%5BEEMDHRHA203%5D%20%28hum%20Airvo2%29%20HOSPITAL%20POLE%20STAND%20900PT421?unique=9ff5f5d)
![[EEMDHRHC201] (hum Airvo2) NASAL CANNULA, optiflow+ adult small OPT942](/web/image/product.template/579118/image_256/%5BEEMDHRHC201%5D%20%28hum%20Airvo2%29%20NASAL%20CANNULA%2C%20optiflow%2B%20adult%20small%20OPT942?unique=e281b81)
![[EEMDHRHC202] (hum Airvo2) NASAL CANNULA, optiflow+ adult medium OPT944](/web/image/product.template/579119/image_256/%5BEEMDHRHC202%5D%20%28hum%20Airvo2%29%20NASAL%20CANNULA%2C%20optiflow%2B%20adult%20medium%20OPT944?unique=e281b81)
![[EEMDHRHC203] (hum Airvo2) NASAL CANNULA, optiflow+ adult large OPT946](/web/image/product.template/579120/image_256/%5BEEMDHRHC203%5D%20%28hum%20Airvo2%29%20NASAL%20CANNULA%2C%20optiflow%2B%20adult%20large%20OPT946?unique=04617cd)
![[EEMDHRHC204] (hum Airvo2) NASAL CANNULA optiflow Jr infant OPT316](/web/image/product.template/579121/image_256/%5BEEMDHRHC204%5D%20%28hum%20Airvo2%29%20NASAL%20CANNULA%20optiflow%20Jr%20infant%20OPT316?unique=e281b81)
![[EEMDHRHC205] (hum Airvo2) NASAL CANNULA optiflow Jr paed OPT318](/web/image/product.template/579122/image_256/%5BEEMDHRHC205%5D%20%28hum%20Airvo2%29%20NASAL%20CANNULA%20optiflow%20Jr%20paed%20OPT318?unique=f128381)
![[EEMDHRHC206] (hum Airvo2) TUBE AND CHAMBER KIT 900PT561](/web/image/product.template/579123/image_256/%5BEEMDHRHC206%5D%20%28hum%20Airvo2%29%20TUBE%20AND%20CHAMBER%20KIT%20900PT561?unique=04617cd)
![[EEMDHRHC207] (hum Airvo2) MASK INTERFACE ADAPTER OPT980](/web/image/product.template/579124/image_256/%5BEEMDHRHC207%5D%20%28hum%20Airvo2%29%20MASK%20INTERFACE%20ADAPTER%20OPT980?unique=66b3f13)
![[EEMDHRHC208] (hum Airvo2) TRACHEOSTOMY INTERFACE optiflow + OPT970](/web/image/product.template/579129/image_256/%5BEEMDHRHC208%5D%20%28hum%20Airvo2%29%20TRACHEOSTOMY%20INTERFACE%20optiflow%20%2B%20OPT970?unique=66b3f13)
![[EEMDHRHC209] (hum Airvo2) TUBE AND CHAMBER KIT Optiflow Junior 900PT531](/web/image/product.template/579131/image_256/%5BEEMDHRHC209%5D%20%28hum%20Airvo2%29%20TUBE%20AND%20CHAMBER%20KIT%20Optiflow%20Junior%20900PT531?unique=66b3f13)
![[EEMDHRHC210] (hum Airvo2) TUBE AND CHAMBER KIT Optiflow/+ 900PT501](/web/image/product.template/579132/image_256/%5BEEMDHRHC210%5D%20%28hum%20Airvo2%29%20TUBE%20AND%20CHAMBER%20KIT%20Optiflow-%2B%20900PT501?unique=66b3f13)
![[EEMDHRHC211] (hum Airvo2) NASAL CANNULA optiflow Jr premature OPT312](/web/image/product.template/579133/image_256/%5BEEMDHRHC211%5D%20%28hum%20Airvo2%29%20NASAL%20CANNULA%20optiflow%20Jr%20premature%20OPT312?unique=bf9e276)
![[EEMDHRHC212] (hum Airvo2) NASAL CANNULA optiflow Jr neonatal OPT314](/web/image/product.template/579134/image_256/%5BEEMDHRHC212%5D%20%28hum%20Airvo2%29%20NASAL%20CANNULA%20optiflow%20Jr%20neonatal%20OPT314?unique=bf9e276)
![[EEMDHRHC214] (hum Airvo2) WIGGLEPADS OPT012](/web/image/product.template/579135/image_256/%5BEEMDHRHC214%5D%20%28hum%20Airvo2%29%20WIGGLEPADS%20OPT012?unique=bf9e276)
![[EEMDHRHC215] (hum Airvo2) OXYGEN TUBING OPT014](/web/image/product.template/579136/image_256/%5BEEMDHRHC215%5D%20%28hum%20Airvo2%29%20OXYGEN%20TUBING%20OPT014?unique=3e30a3a)
![[EEMDHRHC216] (hum Airvo2) NASAL CANNULA Optiflow Jr2 XS premature OJR410](/web/image/product.template/579148/image_256/%5BEEMDHRHC216%5D%20%28hum%20Airvo2%29%20NASAL%20CANNULA%20Optiflow%20Jr2%20XS%20premature%20OJR410?unique=c7fbbd2)
![[EEMDHRHC217] (hum Airvo2) NASAL CANNULA Optiflow Jr2 S neonatal OJR412](/web/image/product.template/579149/image_256/%5BEEMDHRHC217%5D%20%28hum%20Airvo2%29%20NASAL%20CANNULA%20Optiflow%20Jr2%20S%20neonatal%20OJR412?unique=c7fbbd2)
![[EEMDHRHC218] (hum Airvo2) NASAL CANNULA Optiflow Jr2 M infant OJR414](/web/image/product.template/579150/image_256/%5BEEMDHRHC218%5D%20%28hum%20Airvo2%29%20NASAL%20CANNULA%20Optiflow%20Jr2%20M%20infant%20OJR414?unique=c361c67)
![[EEMDHRHC219] (hum Airvo2) NASAL CANNULA Optiflow Jr2 L paediatric OJR416](/web/image/product.template/579151/image_256/%5BEEMDHRHC219%5D%20%28hum%20Airvo2%29%20NASAL%20CANNULA%20Optiflow%20Jr2%20L%20paediatric%20OJR416?unique=c7fbbd2)
![[EEMDHRHC220] (hum Airvo2) NASAL CANNULA Optiflow Jr2 XL big child OJR418](/web/image/product.template/579152/image_256/%5BEEMDHRHC220%5D%20%28hum%20Airvo2%29%20NASAL%20CANNULA%20Optiflow%20Jr2%20XL%20big%20child%20OJR418?unique=c7fbbd2)
![[EEMDHRHC221] (hum Airvo2) WIGGLEPADS WJR112](/web/image/product.template/579819/image_256/%5BEEMDHRHC221%5D%20%28hum%20Airvo2%29%20WIGGLEPADS%20WJR112?unique=c7fbbd2)
![[EEMDHRHC222] (hum Airvo2) WIGGLEPADS WJR110](/web/image/product.template/580211/image_256/%5BEEMDHRHC222%5D%20%28hum%20Airvo2%29%20WIGGLEPADS%20WJR110?unique=c7fbbd2)
![[EEMDHRHS201] (hum Airvo2) DISINFECTION KIT 900PT600](/web/image/product.template/579125/image_256/%5BEEMDHRHS201%5D%20%28hum%20Airvo2%29%20DISINFECTION%20KIT%20900PT600?unique=e281b81)
![[EEMDHRHS202] (hum Airvo2) CLEANING SPONGE STICK 900PT602](/web/image/product.template/579126/image_256/%5BEEMDHRHS202%5D%20%28hum%20Airvo2%29%20CLEANING%20SPONGE%20STICK%20900PT602?unique=04617cd)
![[EEMDHRHS203] (hum Airvo2) DISINFECTION FILTER 900PT601](/web/image/product.template/579127/image_256/%5BEEMDHRHS203%5D%20%28hum%20Airvo2%29%20DISINFECTION%20FILTER%20900PT601?unique=04617cd)
![[EEMDHRHS204] (hum Airvo2) AIR FILTER 900PT913](/web/image/product.template/579128/image_256/%5BEEMDHRHS204%5D%20%28hum%20Airvo2%29%20AIR%20FILTER%20900PT913?unique=04617cd)
![[EEMDHRHS205] (hum Airvo2) OXYGEN INLET EXTENSION KIT 900PT422](/web/image/product.template/579130/image_256/%5BEEMDHRHS205%5D%20%28hum%20Airvo2%29%20OXYGEN%20INLET%20EXTENSION%20KIT%20900PT422?unique=04617cd)
![[EEMDHRHS206] (hum Airvo2) FILTER HOLDER 900PT912](/web/image/product.template/579137/image_256/%5BEEMDHRHS206%5D%20%28hum%20Airvo2%29%20FILTER%20HOLDER%20900PT912?unique=3e30a3a)
![[EEMDHRHS209] (hum Airvo2) BASKET 900PT426](/web/image/product.template/579140/image_256/%5BEEMDHRHS209%5D%20%28hum%20Airvo2%29%20BASKET%20900PT426?unique=8ce00e6)
![[EEMDHRHS210] (hum Airvo2) OXYGEN BOTTLE HOLDER 900PT427](/web/image/product.template/579141/image_256/%5BEEMDHRHS210%5D%20%28hum%20Airvo2%29%20OXYGEN%20BOTTLE%20HOLDER%20900PT427?unique=bf9e276)
![[EEMDHRHS211] (hum Airvo2) UPS MOUNTING KIT 900PT411](/web/image/product.template/579142/image_256/%5BEEMDHRHS211%5D%20%28hum%20Airvo2%29%20UPS%20MOUNTING%20KIT%20900PT411?unique=bf9e276)
![[EEMDHRHS212] (hum Airvo2) STORAGE COVER 900PT603](/web/image/product.template/579143/image_256/%5BEEMDHRHS212%5D%20%28hum%20Airvo2%29%20STORAGE%20COVER%20900PT603?unique=4680f95)