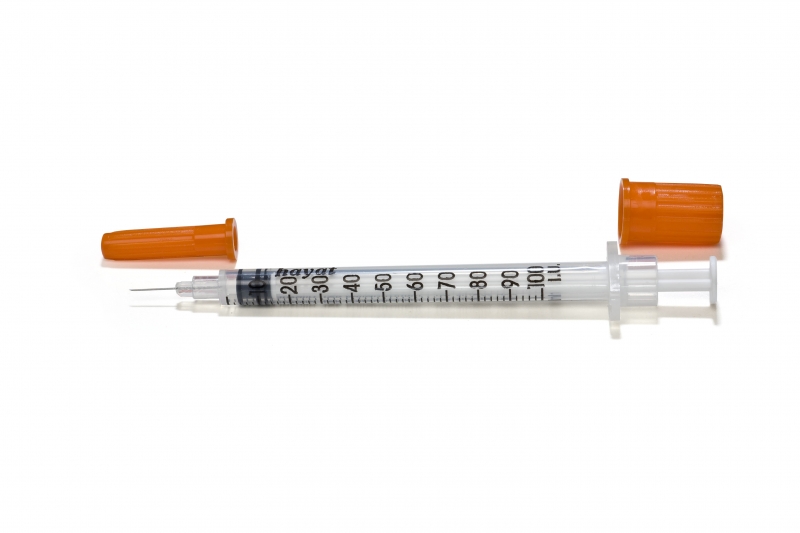
SYRINGE, U-100 insulin, 0.5ml + fixed needle 6-8 mm
STD
SINSSYLIN03
Valid Article
Last Updated on:
07/11/2024, 18:41:57
Former
Code(s):
SINSZTR0005 SINSOCBZGR0011
Single use
A single-use device, also referred to as a disposable device, is intended for use on one patient during a single procedure. It is not intended to be reprocessed (i.e., cleaned and disinfected or sterilized).
A02010601 - Insulin syringes with fixed needle, single-use
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes
Thermosensitive codes are defined for storage and transportation temperature requirements of the products.
INSULIN SYRINGE, Luer, fixed needle
Definition
Sterile three part syringe with fixed needle used for subcutaneous injection of insulin at a concentration of 100 IU/ml.
Specifications
Type of insulin syringe (according ISO 8537): Type 8 = syringe with fixed needle tube and fitted with protective end caps and packaged.
General specifications
- The syringe shall indicate, through visual and non-visual (e.g. tactile) means, the insulin concentration it is intended to contain.
- The syringe scale shall be graduated in increments corresponding to units of only one concentration of insulin.
- The barrels of syringes shall be marked with the insulin concentration for which the syringe was designed to hold, e.g. the text “U-100 insulin” (“units” or “I.U.”)
- The nominal capacity of the syringe shall be designated in millilitres (ml)
- The barrel length shall be such that the syringe has a usable capacity of either 10 % more than the nominal capacity or 3 mm of plunger travel beyond the scale marking, whichever is less.
- The syringe nozzle shall be situated centrally, i.e. shall be co-axial with the barrel.
- The needle length shall be measured as shown and the tolerance of the needle length shall be within ±1,25 mm.
- The dead space shall not exceed 0.01 ml for insulin syringes type 7 and 8
colour coding
- The barrel of the insulin syringe shall be clear, with graduation markings of a colour that contrasts clearly with the syringe.
- No additional colours, other than black and white, shall be used on the syringe barrel.
- For insulin syringes with fixed needles, the colour of the needle cap shall be the colour designated for the insulin concentration. The following colours are given in order to prevent regional variation and to prevent the use of the same colour for different concentrations and different colours for the same concentration: red = U40 / orange = U100 / light blue = U200 / yellow = U300 / purple = U400 / green = U500
graduation scales for U-100 insulin syringes
| nominal capacity | minimum length of scale | scale interval |
| 0.3 ml | 41 mm | 0.5 IU or 1 IU |
| 0.5 ml | 43 mm | 1 IU |
| 1.0 ml | 57 mm | 1 IU or 2 IU |
- The graduation lines shall be of a uniform thickness between 0.2 mm and 0.4 mm
- When the syringe is held vertically, the ends of all graduation lines of similar length shall be vertically aligned with the axis of the barrel and with each other, within a tolerance of ±0,5 mm.
- The length of the short graduation lines shall be approximately half the length of the long graduation lines.The graduation lines shall be numbered: at every five units for the 0.3 ml and 0.5 ml syringes, and at every 10 units for the 1.0 ml syringes.
- The height of the numbers should be at least 3 mm. The numbers shall appear upright on the scale and centred on the graduation lines to which they relate. The numbers shall be close to, but shall not touch, the ends of the graduation lines to which they relate.
Quality standards
ISO 8537, 2016, edition 3, (confirmed 2021) Sterile single-use syringes, with or without needle, for insulin
Technical specifications
- BARREL
- transparent polypropylene
- capacity: 0.5 or 1 ml
- central nozzle
- with flanges: "wings" that jut out from the side of the syringe barrel. they provide an area or surface for the index finger and the middle finger to grasp during aspiration or administration.
- easily read graduations expressed in IU:
- syringe 0.5 ml: every unit
- syringe 1ml: every 1 - 2 units
- indicated insulin concentration: 100 IU/ml
- PLUNGER
- polypropylene, polyethylene
- perfectly fit to slide easily and smoothly inside the barrel
- with plunger cap
- GASKET
- synthetic elastomer (latex free)
- ensures an efficient seal
- NEEDLE
- 29 - 31 G (0.337 - 0.261 mm nominal outer diameter)
- length: 6 - 8 mm
- stainless steel
- mounted on the syringe, not retractable
- with protective needle cap
- Sterile, for single use
Packaging & Labelling
Unit sterile packaging in peel-open pack
Instructions for use
Preparation for administration:
- Wash hands.
- Check the insulin vial (type and strength of insulin, expiry date) and the solution (clear, colourless and aqueous).
- Use an insulin syringe with fixed needle: 1 ml syringe graduated in 2 IU or 0.5 ml syringe graduated in 1 IU (intended for children).
- Stir the insulin by gently rolling the vial between the palms of the hands.
- Pull air into the syringe and inject air into the vial.
- Draw up the required dose of insulin into the syringe.
How to administer SC insulin:
- Insulin can be injected into the abdomen (two fingers away from the belly button), upper arm, buttocks, hips, buttocks, or the front or side of the thigh.
- Injecting insulin in the same area repeatedly can cause lumps, swelling and thickened skin, and it may keep insulin from absorbing properly. See the picture below on “rotating” injection sites.
- Do not inject insulin into areas that have wounds or bruising.
- Clean the chosen injection site with soap and water. Allow to dry.
- Pinch the skin.
- Hold the syringe like a pencil at a 90-degree angle to the skin and insert the needle with one quick motion.
- Inject the dose of insulin. Wait for 5 seconds before removing the needle from the skin.
- Apply gentle pressure on the injection site with the finger for 5-10 seconds to keep insulin from leaking out.
- Do not recap the syringe. Safety discard the needle into a sharps container.
Some restricted information has been hidden. Sign in
to see this information
Precautions for Use
To prevent accidental needlestick injuries, never recap the needle after use.
Some restricted information has been hidden. Sign in
to see this information