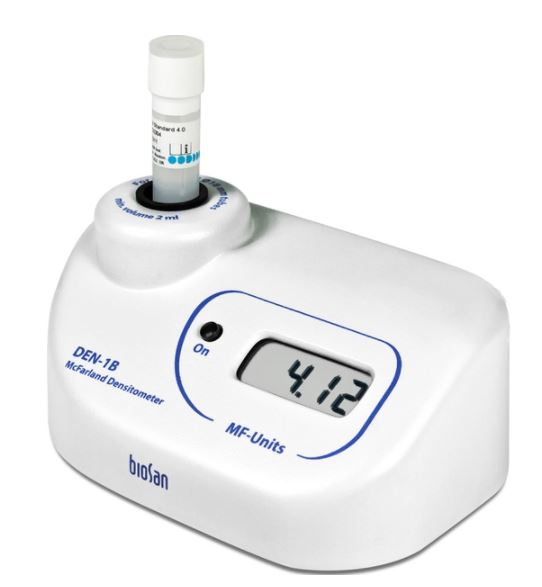
DENSITOMETER Biosan DEN-1B
STD
ELAEDENE3--
Valid Article
HS Code:
901890
Last Updated on:
13/11/2025, 22:20:14
W020105010203 - Densitometers
European Medical Device Nomenclature (EMDN) is the nomenclature of use by manufacturers when registering their medical devices in the EUDAMED database. EMDN is characterised by its alphanumeric structure that is established in a seven-level hierarchical tree.
Thermosensitive codes are defined for storage and transportation temperature requirements of the products.
The product is part of at least one Kit.
A kit is a collection of products (medical and/or logistic) that are needed for a certain intervention in emergency. The choice and quantity of the articles reflects the MSF protocols for this specific situation. The use of Kits allows to start an intervention without a detailed evaluation.
DENSITOMETER Biosan DEN-1B
Definition
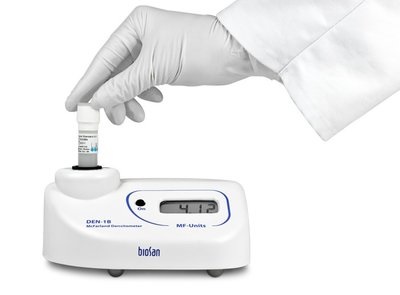
A densitometer is designed to measure the optical density of a bacterial inoculum produced in an tube of liquid medium.
Specifications
Some restricted information has been hidden. Sign in
to see this information
Technical specifications
- Measurement range: 0.00–6.00 McF (possibility to measure up to 15.00 McF with measurement error increase)
- Display resolution: 0.01 McF
- Light source: LED
- Measurement wavelength (λ): λ = 565 ±15 nm
- Accuracy (0.00–6.00 McF): ±3%
- Measurement time: 1 s
- Sample volume: not less than 2 ml
- Tube external diameter: 12 mm, 16 mm (using A-12, A-16 adapter) or 18 mm (without adapter)
- Possibility to restore factory calibration settings
- Display: LCD
- Independent power supply: 3 × AA batteries
- Input current/power consumption: 12 V, 7 mA / 0.1 W
- External power supply: Input AC 100–240 V, 50/60 Hz; Output DC 12 V
- to use between 4 to 40°C
- Standard set: Densitometer DEN-1B, external power supply, A-16 adapter, 3 × AA batteries, operating manual and declaration of conformity
Dimensions
Dimensions:165x115x75 mm
Weight: 0.7 kg
To be Ordered Separately
- Calibration kit
Instructions for use
Follow the recommendations of the user manual.
Please consult the “Bacteriology laboratory procedures and resources” available online via the Laboratory working Group sharepoint page: Laboratory Procedures and Resources
For offline access, contact your laboratory advisor.
Precautions for Use
- In case of use with AC adaptor, use an emergency power supply - UPS.
- All biological fluids should be considered as potentially infectious and handled with the usual precautions (compulsory wearing of gloves, hand washing, etc.).
Maintenance
The maintenance of the optical block must be carried out once a month, or after a cleaning of the equipment.
MSF requirements
Reserved for projects using the Mini-Lab and bacteriology programmes.
Some restricted information has been hidden. Sign in
to see this information






![[ELAEDENC302] (densitometer Biosan) CALIBRATION KIT SD2350](/web/image/product.template/578530/image_256/%5BELAEDENC302%5D%20%28densitometer%20Biosan%29%20CALIBRATION%20KIT%20SD2350?unique=0930dc2)
![[ELAEDENE11-] DENSITOMETER DENSIMAT, with batteries [BMX-99234]](/web/image/product.template/569834/image_256/%5BELAEDENE11-%5D%20DENSITOMETER%20DENSIMAT%2C%20with%20batteries%20%5BBMX-99234%5D?unique=68a2479)
![[SLASWAIN1V03] INOCULUM WATER, 3 ml vial (MicroScan B1015-2)](/web/image/product.template/578511/image_256/%5BSLASWAIN1V03%5D%20INOCULUM%20WATER%2C%203%20ml%20vial%20%28MicroScan%20B1015-2%29?unique=277c912)
![[KMEDMBLB014] (bacteriology Mini-Lab) M4 - INCUBATION MODULE](/web/image/product.template/579044/image_256/%5BKMEDMBLB014%5D%20%28bacteriology%20Mini-Lab%29%20M4%20-%20INCUBATION%20MODULE?unique=c2b20f0)