PERITONEAL DIAGNOSTIC LAVAGE SET, ster. s.u.
Valid Article
PERITONEAL DIAGNOSTIC LAVAGE SET
Definition
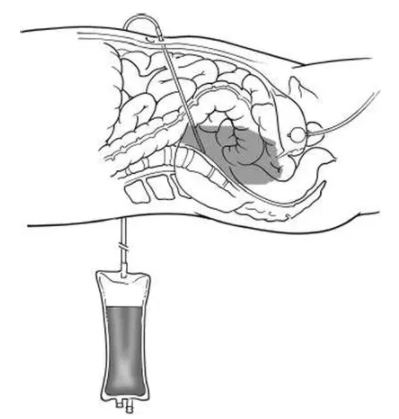
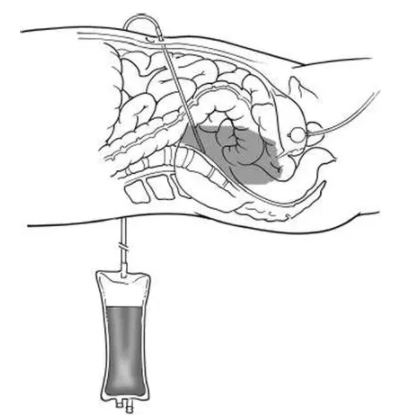
The Diagnostic Peritoneal Lavage (DPL) set contains a specific catheter needed to instill fluid into the peritoneal cavity that will be drained afterwards for analysis. The set includes some material used for the catheter placement using the Seldinger method. DPL is an invasive emergency procedure used to detect hemoperitoneum and help determine the need for laparotomy.
Specifications
Quality standards
Technical specifications
Components of the set:
- Peritoneal lavage catheter
- 90 sideports
- CH 09, length = 20 cm
- catheter material is radiopaque (x-ray visualization)
- Percutaneous entry needle
- 18G, length = 7 cm
- Straight fixed core wire guide
- PFTE coated
- Ø 0.9 mm, length = 40 cm
The set is sterile and for single use
Packaging & Labelling
Each set is packed in a disposable peel pack allowing safe handling, and storage until needed for use and facilitating proper aseptic presentation.
Instructions for use
A Foley catheter and a nasogastric tube are inserted to decompress the bladder and stomach.
After the application of local anaesthesia, a vertical skin incision is made one third of the distance from the umbilicus to the pubic symphysis. A catheter is inserted towards the pelvis and aspiration of material attempted using a syringe. If no blood is aspirated, 1 litre of 0.9% saline is infused and after a few (usually 5) minutes this is drained and sent for analysis. Adequate fluid analysis requires at least 30% of the original amount infused.
Microscopic examination of lavage fluid may refine the diagnostic value, rather than merely characterizing the colour of the fluid as “salmon pink”
Precautions for Use
Use reserved for doctors properly trained in diagnostic peritoneal lavage.