(C-arm) PROTECTION COVER IMAGE INTENSIFIER,60x100cm s.u.ster
STD
EDIMXRFC102
Valid Article
HS Code:
901890
Last Updated on:
13/08/2025, 22:14:37
Single use
A single-use device, also referred to as a disposable device, is intended for use on one patient during a single procedure. It is not intended to be reprocessed (i.e., cleaned and disinfected or sterilized).
CE marking: declaration that the product meets EU standards for health, safety, and environmental protection. The CE marking indicates that the product may be sold freely in any part of the European Economic Area, regardless of its country of origin.
In Europe, medical material that fulfills the definition of a medical device according to the MDR (Medical Device Regulation) is classified into 4 classes
(fluoroscopic x-ray) PROTECTION COVER C-Arm, s.u., sterile
Definition
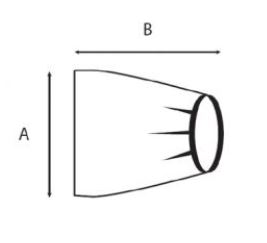
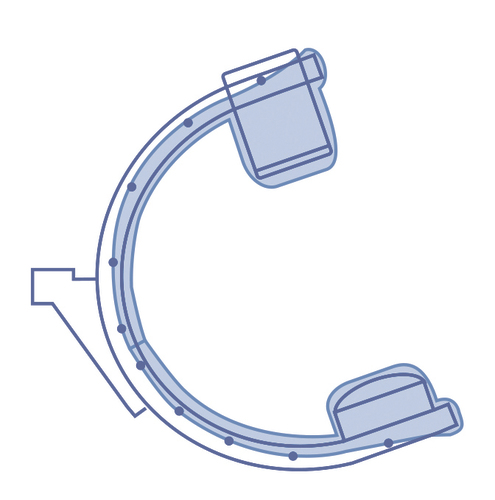
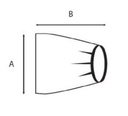
Sterile, single-use plastic protective cover for fluoroscopic x-ray system (C-arm) to protect them from body fluids, blood and contaminants
Specifications
Technical specifications
- highly resistant polyethylene
- antistatic
- transparent
- sterile, single use
- different sizes according the part to cover:
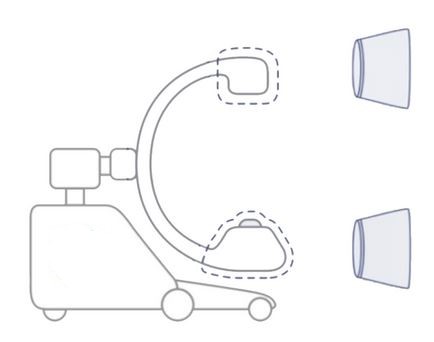
- EDIMXRFC103: complete set including 3 covers for the 3 parts
- 2 banded bag covers (min 100x60 cm)
- 1 C-arm cover
- EDIMXRFC102:
- for part 1 (image intensifier or flat detector) or part 3 (x-ray tube)
- minimum 100 x 60 cm
- elastic fixation/ banded bag
Packaging & Labelling
Varying according the manufacturer: box of 20, 40, 60, 80...
MSF requirements
Sterile cover for orthopaedic interventions: internal fixations.
Some restricted information has been hidden. Sign in
to see this information
Some restricted information has been hidden. Sign in
to see this information

![[EDIMXRFE2--] FLUOROSCOPY SYSTEM, mobile, digital (Cios Select VA21 II)](/web/image/product.template/570540/image_256/%5BEDIMXRFE2--%5D%20FLUOROSCOPY%20SYSTEM%2C%20mobile%2C%20digital%20%28Cios%20Select%20VA21%20II%29?unique=277d91d)