FLUOROSCOPY SYSTEM, mobile, digital (Cios Select VA21 II)
Valid Article
FLUOROSCOPY SYSTEM, mobile, digital (Cios Select VA21 II)
For technical guidance when ordering a fluoroscopic X-ray system (C-arm) contact the Diagnostic Imaging Working Group/DIWG (diagnostic-network@msf.org).
Definition
A digital mobile fluoroscopic x-ray system (C-arm) is an imaging device which uses X-rays to produce a live image which is displayed on a monitor. It is used inside an operating theatre when intraoperative fluoroscopic guidance is required, for example in orthopaedic internal fixation surgery.
Synonym
C-arm, image intensifier
Specifications
The C-arm should be clinically versatile, have excellent image quality with a low radiation dose and be cost-effective. It should be suitable for use with orthopaedic procedures in the operating theatre. All technical specifications to be discussed with the Diagnostic Imaging Working Group.
Quality standards
- EN 60601-2-43, 2022, edition 3, Medical electrical equipment - Part 2-43: Particular requirements for basic safety and essential performance of X-ray equipment for interventional procedures
- IEC 60825-1, 2014, edition 3, Safety of laser products - Part 1: Equipment classification and requirements
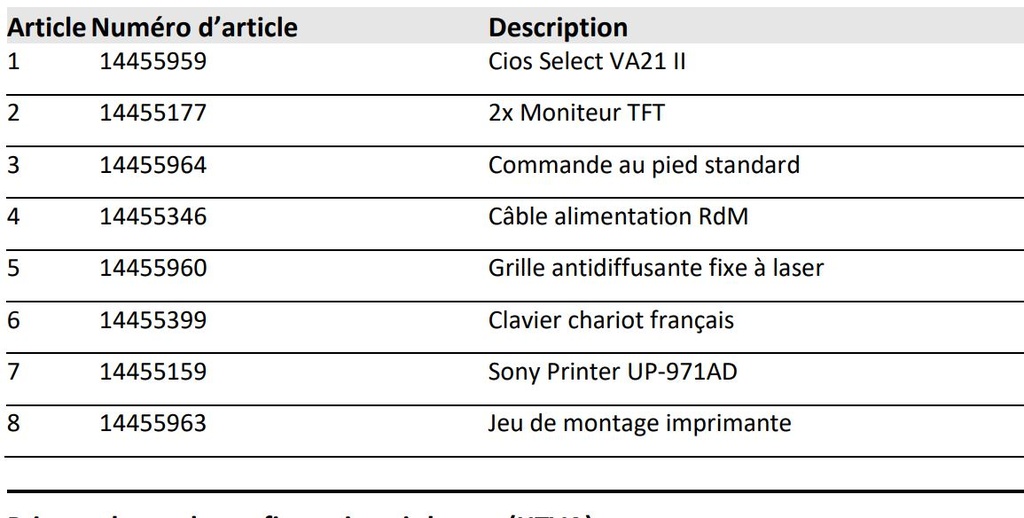
Components
- Cios Select VA21 II
- 2x TFT monitor
- Standard foot switch
- RdM power cable
- Fixed laser anti-glare grille
- US cart keyboard
- Sony Printer UP-971AD
- Printer mounting kit
Technical specifications
C-arm with mechanical breaks:
- Orbital movement 130 ° (- 40 ° to + 90 °)
- Angulation ± 190 °
- Horizontal movement 20 cm (7.9”)
- Immersion depth 73 cm (28.7”)
- Swivel range ± 12 °
- Vertical travel 42 cm (16.5”), motorized
- Source to image-receptor distance 100 cm (39.4”)
- 7 m power cable
X-ray generator:
- Nominal peak output power 2.5 kW
- kV range 40 kV to 110 kV
- Single Image 0.2 mA to 13 mA
- Continuous fluoroscopy 0.2 mA to 13 mA
- Pulsed fluoroscopy 3 mA to 24 mA
Monitor cart:
- 19" (48 cm) diagonal sized twin monitors
- 300 cd/m3 or greater brightness
- DICOM image storage and export
- DVD and USB image export
- Paper printer
- 8.5 m monitor cart cable
Power Supply:
- Power requirements 100 V, 110 V, 120 V, 127 V, 200 V, 230 V, 240 V, (± 10 %), 50/60 Hz (± 1 Hz)
- Maximum power consumption 4.6 kW
- Standby power consumption (for 230 V) 713 W
- Internal line impedance
- Ri max. 0.3 ohms for 100 V to 127 V
- Ri max. 0.8 ohms for 200 V to 240 V
Environmental operating conditions:
- Temperature range + 10 °C to + 35 °C
- Relative humidity 20% to 75%
- Barometric pressure 700 hPa to 1060 hPa
Dimensions
Dimensions and weight:
- Chassis: (l x w x h) 179.5 cm x 80 cm x 173.5 cm. 275 kg
- Monitor cart: (l x w x h) 68.5 cm x 74 cm x 179 cm. 150 kg
Instructions for use
Precautions for Use
Should only be used in a OT with suitable environmental radiation protection measures in place.
- 1W RF 800MHz-2.5GHz TFX min distance of 2.5 meters away; IPX3 waterproof xray tube and c-arm;
- floors should be made of wood, concrete or ceramic tiles and bear the 300kg weight on 4 wheels.
- if the floor is covered with synthetic materials, the relative humidity must be at least 30%.
- during transport floor inclination can not exceed 10 degrees
It is mandatory to protect the device with a double conversion UPS (see related articles below).
Full wrap around radiation safety gowns and thyroid protection, or a mobile radiation barrier must be used by all staff in the OT during operation of the C-arm.
Dedicated training on the equipment is required prior to use. Please contact the DIWG to arrange.
Maintenance
A service contract MUST be arranged with the manufacturer at the time of purchase. In addition there are specific maintenance requirements at project level. Please see MSF X-ray SharePoint - X-ray equipment maintenance folder, and the X-ray user maintenance SOPs document.
MSF requirements
Article for surgical programmes when intraoperative fluoroscopic guidance is required during surgical interventions.
Installation MUST be accompanied by an experienced radiographer training.






![[EDIMAPRO2L-] RADIATION SHIELDING SKIRT+ VEST, 0.5/0.25mm Pb equivalent, L](/web/image/product.template/569067/image_256/%5BEDIMAPRO2L-%5D%20RADIATION%20SHIELDING%20SKIRT%2B%20VEST%2C%200.5-0.25mm%20Pb%20equivalent%2C%20L?unique=3fb798d)
![[EDIMAPRO2M-] RADIATION SHIELDING SKIRT+ VEST, 0.5/0.25mm Pb equivalent, M](/web/image/product.template/569107/image_256/%5BEDIMAPRO2M-%5D%20RADIATION%20SHIELDING%20SKIRT%2B%20VEST%2C%200.5-0.25mm%20Pb%20equivalent%2C%20M?unique=7092ca5)
![[EDIMAPRO2S-] RADIATION SHIELDING SKIRT+ VEST, 0.5/0.25mm Pb equivalent, S](/web/image/product.template/569108/image_256/%5BEDIMAPRO2S-%5D%20RADIATION%20SHIELDING%20SKIRT%2B%20VEST%2C%200.5-0.25mm%20Pb%20equivalent%2C%20S?unique=3fb798d)
![[EDIMAPRO2XL] RADIATION SHIELDING SKIRT+ VEST, 0.5/0.25mm Pb equivalent,XL](/web/image/product.template/569110/image_256/%5BEDIMAPRO2XL%5D%20RADIATION%20SHIELDING%20SKIRT%2B%20VEST%2C%200.5-0.25mm%20Pb%20equivalent%2CXL?unique=7092ca5)
![[EDIMCOLL1--] RADIATION SHIELDING COLLAR, 0.5 mm Pb equivalent, w/collar](/web/image/product.template/570331/image_256/%5BEDIMCOLL1--%5D%20RADIATION%20SHIELDING%20COLLAR%2C%200.5%20mm%20Pb%20equivalent%2C%20w-collar?unique=d0298c3)
![[EEMDTAOA301] ORTHOPEDIC TRACTION SYS ORT5000C for op. table Surginox](/web/image/product.template/571748/image_256/%5BEEMDTAOA301%5D%20ORTHOPEDIC%20TRACTION%20SYS%20ORT5000C%20for%20op.%20table%20Surginox?unique=0c94c00)
![[EEMDTAOE21-] OPERATING TABLE, mechanical / hydraulic (Surginox ST4080AMH)](/web/image/product.template/571992/image_256/%5BEEMDTAOE21-%5D%20OPERATING%20TABLE%2C%20mechanical%20-%20hydraulic%20%28Surginox%20ST4080AMH%29?unique=1ec55f7)
![[KPROMPOSUA06X] MODULE UPS double convers. (Delta RT EXTEND.) 6kVA, 1ph/1ph](/web/image/product.template/577879/image_256/%5BKPROMPOSUA06X%5D%20MODULE%20UPS%20double%20convers.%20%28Delta%20RT%20EXTEND.%29%206kVA%2C%201ph-1ph?unique=be2ad6c)
![[EDIMXRFC102] (C-arm) PROTECTION COVER IMAGE INTENSIFIER,60x100cm s.u.ster](/web/image/product.template/580445/image_256/%5BEDIMXRFC102%5D%20%28C-arm%29%20PROTECTION%20COVER%20IMAGE%20INTENSIFIER%2C60x100cm%20s.u.ster?unique=4d00ed0)
![[EDIMXRFC103] (C-arm) COMPLETE PROTECTION COVER 3 parts, s.u., sterile](/web/image/product.template/580444/image_256/%5BEDIMXRFC103%5D%20%28C-arm%29%20COMPLETE%20PROTECTION%20COVER%203%20parts%2C%20s.u.%2C%20sterile?unique=4d00ed0)
![[EDIMXRFS202] (C-Arm Cios Select) SG CABLE FEMALE SIDE, 10893434](/web/image/product.template/575284/image_256/%5BEDIMXRFS202%5D%20%28C-Arm%20Cios%20Select%29%20SG%20CABLE%20FEMALE%20SIDE%2C%2010893434?unique=abfc26b)
![[EDIMXRFS203] (C-Arm Cios Select) GRAPHIC CARD K2200, 11104920](/web/image/product.template/575285/image_256/%5BEDIMXRFS203%5D%20%28C-Arm%20Cios%20Select%29%20GRAPHIC%20CARD%20K2200%2C%2011104920?unique=abfc26b)