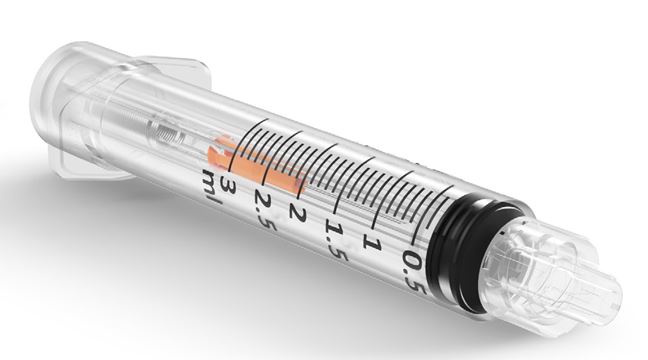
SYRINGE, Re-Use Prev., Luer, automat. Retract., 1 ml (SafeR)
Valid Article
SYRINGE, Re-Use Prevention, retractable, SafeR
Definition
SafeR® is an automatically Retractable Safety Syringe, it is intended to provide a safe, accurate and reliable method for the injection of fluids and medications.
The syringe, once assembled with its needle (SafeR® Sting), incorporates a passive safety mechanism which retracts and contains the contaminated needle after use aiding in the prevention of possible infections due to needle stick injuries and syringe reuse. Needle retraction is activated by the syringe user with one hand, with one just click.
Specifications
The definition of RUP syringes includes syringes that can measure flexible dosing amounts, have removable needles and a feature that blocks the syringe from being used a second time.
This definition is provided for syringes used in curative services where a broad range of injection procedures are performed and are typically 1.0 – 10.0 ml in size.
SIPs cover AD and RUP syringes that have an additional feature to prevent sharps injury, such as a means to contain the infected sharp after use.
- SIP = sharp (needle stick) injury prevention
- RUP = re-use prevention
- AD = auto-disable
Quality standards
- ISO 7886-4, 2018, edition 2, Sterile hypodermic syringes for single use - Part 4: Syringes with re-use prevention feature
- ISO 23908, 2013, edition 1, Sharps injury protection - Requirements and test methods - Sharps protection features for single-use hypodermic needles, introducers for catheters and needles used for blood sampling
Technical specifications
RUP + SIP syringe type B: the mechanism allows multiple plunger aspirations if reconstitution of the medication or the vaccine is required, or if multiple drugs need to be mixed in the same syringe before being administered to the patient.
- 3-piece syringe: barrel, plunger, plunger seal
- central tip, Luer-lock type (but not compatible with other Luer-lock devices)
- graduation: / 0.1 ml
- available in sizes: 1ml, 2ml, 3ml, 5ml.
- sterile device, for single use only
Packaging & Labelling
Unit sterile packaging in peel-open pack
Instructions for use
- Plan safe handling and disposal before any procedure
- Use safe and effective needle alternatives when available
- Immediately dispose of contaminated needles in a sharps container
Storage
Shelf life 3 years.
No special storage or transportation condition. Recommendations to store in room temperature. Store in dry and warm place and not exposed to strong light.
MSF requirements
Allows injections using devices with a sharp injury prevention (SIP) and RUP, important in VHF context.


![[KMEDMEBO08-] (module VHF isolation) MEDICAL SUPPLIES](/web/image/product.template/571731/image_256/%5BKMEDMEBO08-%5D%20%28module%20VHF%20isolation%29%20MEDICAL%20SUPPLIES?unique=fb9a656)
![[SINSNEEDR21] NEEDLE, s.u., Luer, retractable, 21Gx38 mm, green (SafeR)](/web/image/product.template/580079/image_256/%5BSINSNEEDR21%5D%20NEEDLE%2C%20s.u.%2C%20Luer%2C%20retractable%2C%2021Gx38%20mm%2C%20green%20%28SafeR%29?unique=921eba6)
![[SINSNEEDR23] NEEDLE, s.u., Luer, retractable, 23Gx32 mm, blue (SafeR)](/web/image/product.template/580080/image_256/%5BSINSNEEDR23%5D%20NEEDLE%2C%20s.u.%2C%20Luer%2C%20retractable%2C%2023Gx32%20mm%2C%20blue%20%28SafeR%29?unique=921eba6)
![[SINSNEEDR25] NEEDLE, s.u., Luer, retractable, 25Gx25 mm, orange (SafeR)](/web/image/product.template/580081/image_256/%5BSINSNEEDR25%5D%20NEEDLE%2C%20s.u.%2C%20Luer%2C%20retractable%2C%2025Gx25%20mm%2C%20orange%20%28SafeR%29?unique=921eba6)