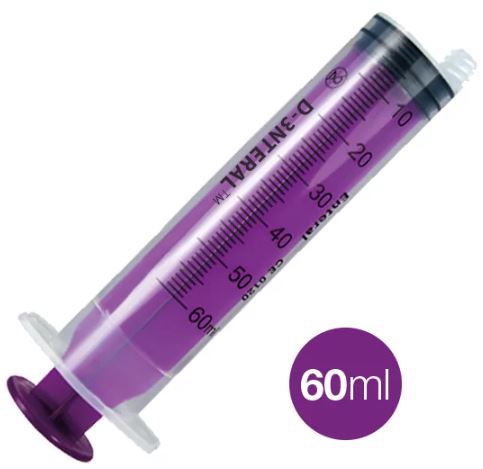
ENTERAL SYRINGE ENFit, 60 ml, washable
Valid Article
ENTERAL SYRINGE, ENFit, washable
Definition
A device intended to be used for the administration of oral medication or delivery of enteral nutrition by connection to an enteral administration device.
It consists of a calibrated hollow barrel (cylinder) made of plastic, and a moveable plunger. The tip is designed to mate only with enteral administration devices and is specifically incompatible with Luer (6%) connectors and intravenous devices.
Specifications
See information on nasogastric and enteral tubes
Quality standards
Technical specifications
washable syringe allowing a reuse for the same person
3-part syringe: barrel + gasket + plunger
To avoid dosage errors with the large dead space of the regular ENFit syringes, a Low Dose Tip (LDT) was developed.
BARREL
- transparent polypropylene
- capacity:
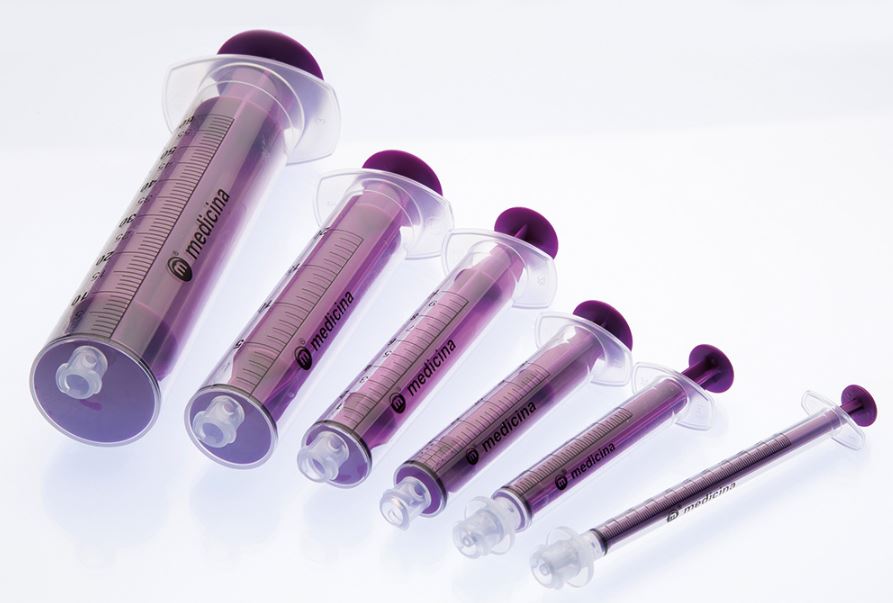
- regular ENFit: 10 ml, 20 ml and 60 ml
- ENFit LDT: 1ml, 2.5 - 3 ml and 5 ml
- easily read graduations
- 1 ml and 2.5 ml syringe => every 0.1 ml
- 5 ml syringe => every 0.2 ml
- 10 ml and 20 ml syringe => every 0.5 ml
- 60 ml syringe => every 1 ml
- ending with a female EnFit tip, LDT (low dose tip) for the syringes 1 - 5 ml to decrease the dead space.
PLUNGER
- polypropylene, polyethylene
- perfectly fit to slide easily and smoothly inside the barrel
- purple colour to distinguish from injection syringes.
GASKET
- synthetic elastomer (latex free)
- ensures an efficient seal
Packaging & Labelling
Unit packaging
Instructions for use
Enteral-specific syringes are introduced with the ENFit female connector. They are required to administer medicine, flush, hydrate, or bolus feed through the ENFit feeding tubes and extension sets.
The small LDT syringes are used to administer oral suspensions and medications via nasogastric tubing.
Cleaning instructions
- do not allow contents to dry in the syringe before washing
- syringes can be cleaned by washing in warm, soapy water
- the plunger should be removed and washed separately
- if visible soil remains, use a soft-bristled brush to aid washing the plunger and the INSIDE of the syringe barrel. Do not use abrasive products on the oiutside of the barrel is this may lead to premature removal of printed markings.
- rinse each component with cold water and allow to air dry
- do not dry the components with paper towel as this will shorten the life of the device
- discard syringe when:
- black O-ring gasket is distorted or damaged
- barrel markings are too difficult to read
- plunger is too difficult to move
Precautions for Use
Oral-tipped and Luer-tipped syringes will not fit the ENFit connector system.
The syringes cannot be sterilised and must be kept single patient use, not to be shared amongst different patients.
Do not use with a syringe driver




![[KMEDMHIS25-] (mod ICU) CATHETERS, TUBES and DRAINS 2021](/web/image/product.template/574344/image_256/%5BKMEDMHIS25-%5D%20%28mod%20ICU%29%20CATHETERS%2C%20TUBES%20and%20DRAINS%202021?unique=d9788e5)
![[KMEDMNUTI33] (module nut. inpatient) MEDICAL SUPPLIES 2021](/web/image/product.template/575289/image_256/%5BKMEDMNUTI33%5D%20%28module%20nut.%20inpatient%29%20MEDICAL%20SUPPLIES%202021?unique=1f49c0c)
![[KMEDMHDS21-] (mod delivery & neonate) RENEWABLE SUPPLIES compl. 2019](/web/image/product.template/569223/image_256/%5BKMEDMHDS21-%5D%20%28mod%20delivery%20%26%20neonate%29%20RENEWABLE%20SUPPLIES%20compl.%202019?unique=8c94843)
![[KMEDMHCS211] (mod OPD) COMPLEMENTARY RENEWABLE SUPPLIES 2021](/web/image/product.template/574350/image_256/%5BKMEDMHCS211%5D%20%28mod%20OPD%29%20COMPLEMENTARY%20RENEWABLE%20SUPPLIES%202021?unique=e7503b9)
![[KMEDMHWS35-] (mod ward) CATHETERS, TUBES & DRAINS 2021](/web/image/product.template/574358/image_256/%5BKMEDMHWS35-%5D%20%28mod%20ward%29%20CATHETERS%2C%20TUBES%20%26%20DRAINS%202021?unique=83796d5)
![[KMEDMSUP04S1] (supplementary unit 2018) MODULE RENEWABLE SUPPLIES 2019](/web/image/product.template/569226/image_256/%5BKMEDMSUP04S1%5D%20%28supplementary%20unit%202018%29%20MODULE%20RENEWABLE%20SUPPLIES%202019?unique=dcc4b7f)
![[KMEDMCHO021] (module 001) RENEWABLE SUPPLIES 2019](/web/image/product.template/569222/image_256/%5BKMEDMCHO021%5D%20%28module%20001%29%20RENEWABLE%20SUPPLIES%202019?unique=904b96e)