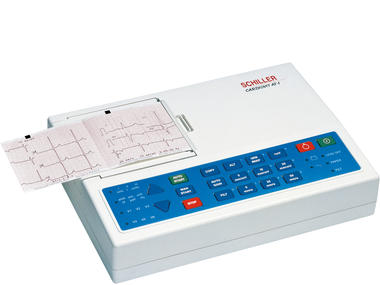
ELECTROCARDIOGRAPH (Schiller AT-1), portable, 3 channels+ACC
Discontinued Article
ELECTROCARDIOGRAPH, portable, Schiller AT-1
Stopped in 2017 because no longer produced and replaced by the new model Schiller AT-1 G2 EEMDECGE4-- (see“replaced by” at bottom of the page).
Definition
Apparatus allowing to record and to report on a support the cardiac activity . It enables the diagnosis of cardio-vascular diseases.
The Shiller AT-1 ECG is no longer manufactured, it is replaced by the Shiller AT-1 G2
Specifications
Quality standards
Technical specifications
- Sensitivity: 5 /10 / 20 mm/mV, automatic or manual setting
- Automatic or manual recording mode
- Leads: Standard / Cabrera
- Automatic program of the measurements: presentation on 3 channels of all 12 simultaneously acquired standard leads
- ECG amplifier: simultaneous acquisition, synchronised with 9 active electrode signs (= 12 standard leads)
- Myogram (muscle tremor) filter
- Patient input circuitry: fully floating and isolated, defibrillation protected
- Wastage of current at patient <5 μA
- Built-in thermal printer
- Power supply:100-115 / 220-240 VAC , 50/60 Hz
- Power consumption: max. 28 VA
- Built-in rechargeable 12 V battery:
- charging time: 3 hours to 60 % capacity, less than 7 hours to 90% capacity
- a fully charged battery allows 2 hours of normal use
- Dimensions: 290 x 210 x 69 mm
- Weight: 2.9 kg
Packaging & Labelling
Unit packaging
Supplied with the Article
- 10-lead patient cable with banana plugs
- Power cable
- Electrode gel tube (50 ml)
- Precordial electrodes
- Limbs electrodes
- Ream of thermo-reactive, z-folded recording paper
- Instructions leaflet
- Carrying bag
Instructions for use
The manual mode allows the printing in real time of 3 selected leads on the built-in thermal printer.
Before or during the recording, it is possible to select the following parameters:
- Group of leads
- Printing speed
- Sensistivity
- Myogram filter
In the automatic mode, a complete ECG with 12 leads is printed in one of the 2 pre-defined report formats at a sensitivity which can be selected. These formats can be
chosen by the user according the specific needs.
When pushing on the AUTO GAIN button before the recording in automatic mode, the apparatus detects the very important amplitude peaks and adjusts the sensitivity
of the extremity and /or precordial leads to reduce the overwriting of the graphs.
- See: 1.8. SOP ECG
Precautions for Use
- When used or stored, the apparatus must be protected from humidity, dust, direct sunlight or any other heat source
- working temperature: 10° to 40°C
- storage temperature: -10° to 50°C
- Avoid contact with steam or liquids containing acids which could cause irreversible damages to this apparatus
- Do not install the apparatus in the neighbourhood of radiography units
- Contact your biomedical officer in order to see the need to protect the apparatus with a double conversion UPS (see related articles below).
MSF requirements
Lightweight, compact and autonomous apparatus



![[EEMDECGA111] (ECG Schiller AT-1/G2) PATIENT CABLE 10 leads](/web/image/product.template/570859/image_256/%5BEEMDECGA111%5D%20%28ECG%20Schiller%20AT-1-G2%29%20PATIENT%20CABLE%2010%20leads?unique=65d7316)
![[EEMDECGA113] (ECG) SUCTION ELECTRODE 4mm conn., Ø 24mm, blue, 1 piece](/web/image/product.template/572533/image_256/%5BEEMDECGA113%5D%20%28ECG%29%20SUCTION%20ELECTRODE%204mm%20conn.%2C%20%C3%98%2024mm%2C%20blue%2C%201%20piece?unique=6888ffc)
![[EEMDECGA114] (ECG) CLIP ELECTRODES, adult, limb, set 4pcs/colors](/web/image/product.template/569311/image_256/%5BEEMDECGA114%5D%20%28ECG%29%20CLIP%20ELECTRODES%2C%20adult%2C%20limb%2C%20set%204pcs-colors?unique=b9fbc0a)
![[EEMDECGA115] (ECG) SET ELECTRODES for CHILDREN, 6 bulbs and 4 clips](/web/image/product.template/572686/image_256/%5BEEMDECGA115%5D%20%28ECG%29%20SET%20ELECTRODES%20for%20CHILDREN%2C%206%20bulbs%20and%204%20clips?unique=2039697)
![[EEMDECGA402] (ECG Schiller) CARRYING BAG 1183](/web/image/product.template/572057/image_256/%5BEEMDECGA402%5D%20%28ECG%20Schiller%29%20CARRYING%20BAG%201183?unique=70ce758)
![[EEMDECGC101] (ECG Schiller AT-1) RECORDING PAPER, 1pack, 2.157056](/web/image/product.template/570860/image_256/%5BEEMDECGC101%5D%20%28ECG%20Schiller%20AT-1%29%20RECORDING%20PAPER%2C%201pack%2C%202.157056?unique=e11cd1e)
![[EEMDECGC102] (ECG) ELECTRODE GEL, bottle 250 ml](/web/image/product.template/570858/image_256/%5BEEMDECGC102%5D%20%28ECG%29%20ELECTRODE%20GEL%2C%20bottle%20250%20ml?unique=90b697a)
![[EEMDECGE4--] ELECTROCARDIOGRAPH (Schiller AT-1 G2), portable, 3 ch+ACC](/web/image/product.template/573290/image_256/%5BEEMDECGE4--%5D%20ELECTROCARDIOGRAPH%20%28Schiller%20AT-1%20G2%29%2C%20portable%2C%203%20ch%2BACC?unique=23d782d)
![[EEMDECGS101] (ECG Schiller AT-1) BATTERY, 12V, 4.350024](/web/image/product.template/571286/image_256/%5BEEMDECGS101%5D%20%28ECG%20Schiller%20AT-1%29%20BATTERY%2C%2012V%2C%204.350024?unique=843dd77)
![[EEMDECGS102] (ECG Schiller AT-1) PRINTER+ PAPER TRAY ASSY 3.910966](/web/image/product.template/571643/image_256/%5BEEMDECGS102%5D%20%28ECG%20Schiller%20AT-1%29%20PRINTER%2B%20PAPER%20TRAY%20ASSY%203.910966?unique=569df3b)
![[EEMDECGS103] (ECG Schiller AT-1) HOUSING AND KEYBOARD engl., 3.910962](/web/image/product.template/577510/image_256/%5BEEMDECGS103%5D%20%28ECG%20Schiller%20AT-1%29%20HOUSING%20AND%20KEYBOARD%20engl.%2C%203.910962?unique=6d5cb35)