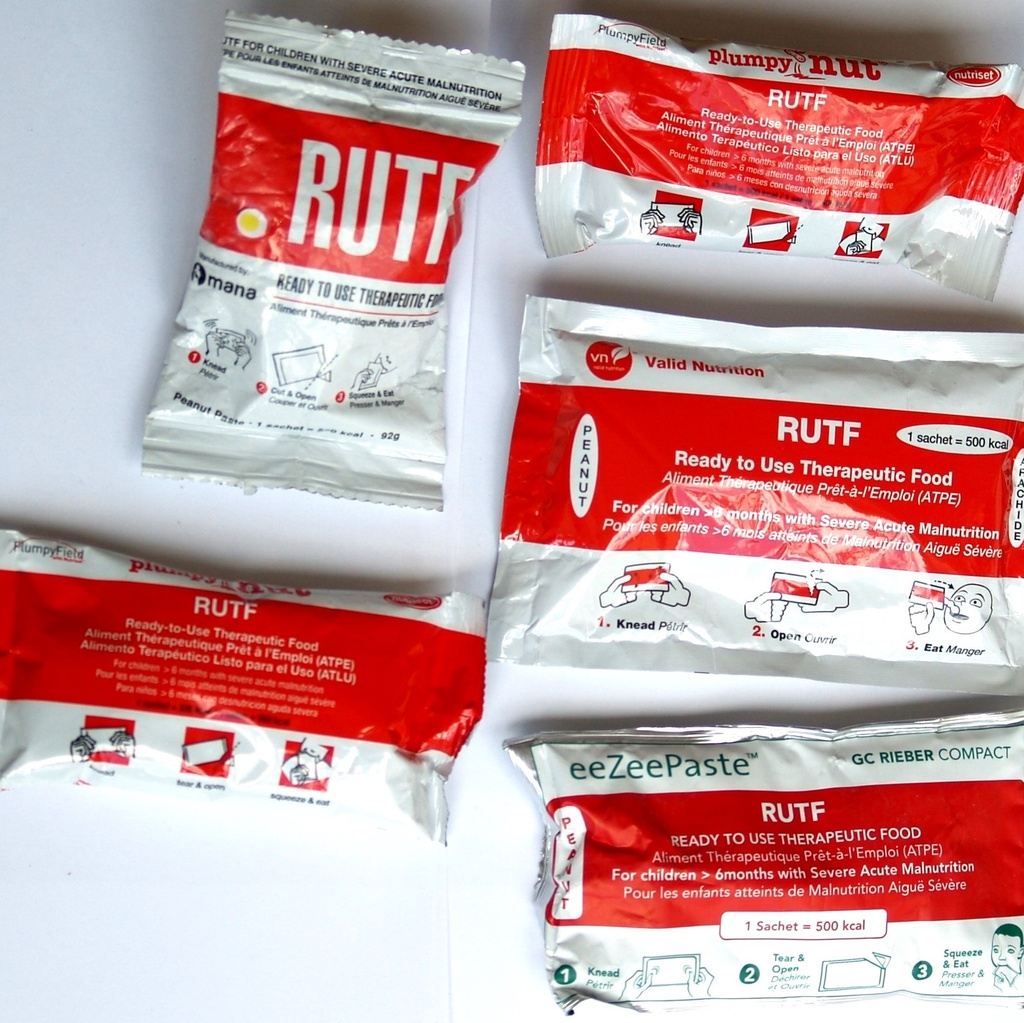
READY-TO-USE THERAPEUTIC FOOD, peanut paste, 92g
Valid Article
RUTF, peanut paste
RUTF - Ready-To-Use Therapeutic Food
Definition
Ready-to-Use Therapeutic Food (RUTF) in paste form is intended for children from 6 months and adults with severe acute malnutrition (SAM) during the rehabilitation phase, in therapeutic feeding centres or in outpatient settings.
Due to its iron content, RUTF should not be used in the initial phase of treatment of severe acute malnutrition.
The nutritional properties of RUTF are similar to those of therapeutic milk, except for its higher iron content.
Examples: Plumpy'nut, eeZeepaste, Imunut, Valid Nutrition, Dutasi, Nutty Butta...
Specifications
Main Composition
Peanut paste, sugar, vegetable fat, dry skimmed milk, whey, malt dextrin, mineral and vitamin complex, natural flavouring, emulsifying agent.
Technical specifications
Nutritional values | Unit | Per 100 g | Per 92 g (dose per sachet) | |
| Min. | Max. | |||
| Energy | kcal | 520 | 550 | 500 |
| Proteins | g | 13 | 16 | 12.8 |
| Lipids | g | 26 | 38 | 30.3 |
| Minerals | ||||
| Calcium (Ca) | mg | 300 | 600 | 302 |
| Phosphorus (P) | mg | 300 | 600 | 243 |
| Potassium (K) | mg | 1100 | 1400 | 1171 |
| Magnesium (Mg) | mg | 80 | 140 | 80 |
| Sodium (Na) | mg | - | < 290 | 165 |
| Iron (Fe) | mg | 10 | 14 | 10.3 |
| Zinc (Zn) | mg | 11 | 14 | 11.8 |
| Copper (Cu) | mg | 1.4 | 1.8 | 1.5 |
| Iodine (I) | µg | 70 | 140 | 98 |
| Selenium (Se) | µg | 20 | 40 | 28 |
| Vitamins | ||||
| Retinol (Vit A) | mg | 0.8 | 1.4 | 0.79 |
| Cholecalciferol (Vit D) | µg | 15 | 20 | 14 |
| Tocopherol (Vit E) | mg | > 20 | - | 18.4 |
| Ascorbic ac. (Vit C) | mg | > 50 | - | 46 |
| Thiamin (Vit B1) | mg | > 0.5 | - | 0.46 |
| Riboflavin (Vit B2) | mg | > 1.6 | - | 1.5 |
| Pyridoxine (Vit B6) | mg | > 0.6 | - | 0.55 |
| Cobalamin (Vit B12) | µg | >1.6 | - | 1.5 |
| Phylloquinone (Vit K) | µg | 15 | 30 | 14.4 |
| Folate (Vit B9) | µg | > 200 | - | 184 |
| Pantothenic ac. (Vit B5) | mg | > 3 | - | 2.8 |
| Biotin (Vit B7) | µg | > 60 | - | 56 |
| Niacin (Vit B3) | mg | > 5 | - | 4.6 |
For more details on specifications (raw material, chemical toxicity...), see MSF Specifications: RUTF peanut paste
Packaging & Labelling
The primary and secondary packaging must protect the hygienic and nutritional qualities of the product during transport, handling, and storage under MSF field conditions.
- Primary packaging (unit packaging): 92 g aluminium sachet
- Secondary packaging: box of 150 sachets
For more information on packaging labelling, refer to the CHECKLIST CONTROL UPON RECEIPT specialised food.
Instructions for use
This product does not require dilution or cooking. Its packaging facilitates direct consumption.
Caution! Do not use in children under 6 months of age.
Do not give to individuals with peanuts or dairy allergies.
If the product is poorly accepted, it can be substituted with Ready-To-Use Therapeutic Food in biscuit form, which offers the same nutritional properties.
Storage
- Below 30º C - Protect from sunlight ‐ Protect from humidity
- Shelf life: between 6 and 24 months from the manufacturing date stated on each sachet.
- After opening, the product should be consumed within 24 hours.
Follow the procedure QA-NFOS-SOP3 Storage of specialised food (NFOS).
For procedures related to Quality Control, refer to Preliminary remarks on specialised food.




![[NSFSCRECOSE] CHECKLIST CONTROL UPON RECEIPT specialised food, English](/web/image/product.template/572035/image_256/%5BNSFSCRECOSE%5D%20CHECKLIST%20CONTROL%20UPON%20RECEIPT%20specialised%20food%2C%20English?unique=c6f8e93)
![[NSFSCRECOSF] CHECKLIST CONTROL UPON RECEIPT specialised food, French](/web/image/product.template/572046/image_256/%5BNSFSCRECOSF%5D%20CHECKLIST%20CONTROL%20UPON%20RECEIPT%20specialised%20food%2C%20French?unique=c6f8e93)
![[KMEDKNUTI3-] KIT, NUTRITION INPATIENT, 50 patients 2021](/web/image/product.template/575120/image_256/%5BKMEDKNUTI3-%5D%20KIT%2C%20NUTRITION%20INPATIENT%2C%2050%20patients%202021?unique=7433b39)
![[KMEDKNUTO3-] KIT, NUTRITION OUTPATIENT, 250 patients 2021](/web/image/product.template/574050/image_256/%5BKMEDKNUTO3-%5D%20KIT%2C%20NUTRITION%20OUTPATIENT%2C%20250%20patients%202021?unique=7433b39)